Abstract
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD), one of the components of Agent Orange, has been reported to be a deadly poison despite its presence at extremely small doses. TCDD is reported to cause various kinds of cancers and other harmful effects on humans. However, a correlation between exposure to TCDD and acute coronary syndrome (ACS) is not yet proven. Thus, we examined the correlation between exposure to TCDD and ACS through an analysis of coronary angiograms from veterans of the Vietnam War. Two hundred fifty-one consecutive men undergoing coronary angiograms owing to ACS between April 2004 and May 2009 at Gwangju Veterans Hospital were analyzed. Included subjects were between 50 and 70 years of age. The patients were divided into two groups: 121 patients who had been exposed to TCDD (Group I) and 130 patients who had not been exposed to TCDD (Group II). Clinical and coronary angiographic findings were evaluated. Baseline clinical characteristics, inflammatory markers, and echocardiographic parameters were not significantly different between the two groups. The incidence of hypertension (71.1% vs. 60.0%, p=0.039) and hyperlipidemia (27.3% vs. 16.9%, p=0.038) was higher in Group I than in Group II. Total occlusion, stent length, stent use, and coronary lesion characteristics were not significantly different between the two groups. The rate of major adverse cardiovascular events (MACE) had no relationship with exposure to TCDD. Exposure to TCDD might not affect severity or the rate of MACE in persons with ACS.
Agent Orange is a herbicide and defoliant that was extensively used by the American military during the Vietnam War to defoliate trees to uncover enemy hideouts in dense jungles. It contains a dioxin, 2,3,7,8-tetrachlorodibenzodioxin (TCDD), that is a member of a large family of halogenated aromatic hydrocarbons and that is reported to be a deadly poison and a carcinogen in humans despite its presence at extremely small doses.1-3
However, whether exposure to TCDD is associated with cardiovascular disease, such as hypertension, hyperlipidemia, diabetes, or ischemic heart disease, or whether it influences severity and adverse events associated with acute coronary syndrome (ACS), including unstable angina, ST-segment elevation myocardial infarction, and non ST-segment elevation myocardial infarction, is unclear.4 Thus, we assessed the possible correlation between exposure to TCDD and cardiovascular disease through an analysis of coronary angiograms. Also, we compared the rates of major adverse cardiac events (MACE) according to exposure to TCDD.
Patients undergoing coronary angiography because of ACS presentation between April 2004 and May 2009 at Gwangju Veterans Hospital were analyzed. We limited the analysis to men between the ages of 50 and 70 years because we considered this to be the age range of Korean veterans in the Vietnam War, which took place from 1961 to 1971. A total of 251 subjects were divided into two groups according to exposure to TCDD (Group I: 121 patients who had been exposed to TCDD, and Group II: 130 patients who had not been exposed to TCDD).
We retrospectively reviewed the medical records of all patients including coronary angiographic findings. We examined the patient's age, body mass index (BMI), cardiovascular disease risk factors (e.g., diabetes, hypertension, hyperlipidemia, and smoking), history of previous percutaneous coronary intervention (PCI), left ventricular ejection fraction (LVEF) by 2 dimensional-echocardiography, and laboratory findings. Significant stenosis was defined as more than 50% stenosis of the inside diameter of involved coronary arteries. Successful PCI was defined as that achieving residual stenosis <30% and thrombolysis in myocardial infarction (TIMI) flow >Grade III without complications, such as cardiac death, acute myocardial infarction, urgent revascularization, or urgent coronary artery bypass graft (CABG). We assessed coronary angiographic findings, procedure-related factors, and adverse events. MACE included death from any cause, myocardial infarction, and symptom-driven revascularization.
Statistical analysis was performed by using SPSS version 12.0. The data are reported as means±SEM for continuous variables and as proportions for categorical variables. A value of p<0.05 was considered significant. Differences between Groups I and II were analyzed by independent t-tests and the chi-square test for continuous and categorical variables, respectively. The rate of MACE was analyzed by performing binary multiple logistic regression analysis.
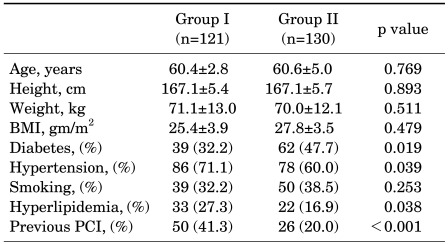
The baseline clinical characteristics of the 251 patients are summarized in Table 1. There were no significant differences in age, BMI, or smoking history between the two groups. The prevalence of hypertension and hyperlipidemia was significantly higher among patients who had been exposed to TCDD (Group I) than among those who had not been exposed, respectively (Group II). Also, the proportion of patients with a history of previous PCI was significantly higher in Group I than in Group II. Conversely, the prevalence of diabetes was significantly higher in Group II than in Group I.
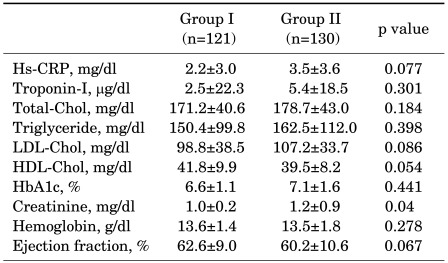
Laboratory findings, including levels of inflammatory makers and cardiac enzymes, and lipid profiles, were not significantly different between the two groups (Table 2). The mean creatinine level was significantly higher in Group II than in Group I, but both remained within the limit of the reference range. LVEF was not significantly different between the two groups.
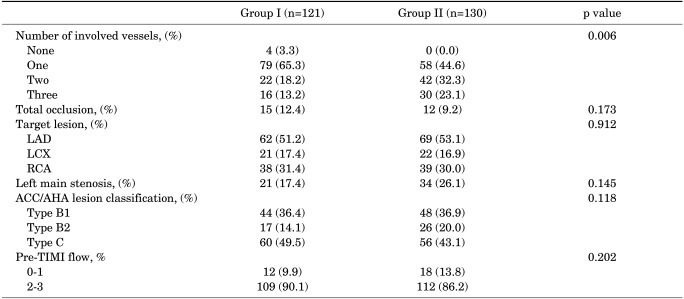
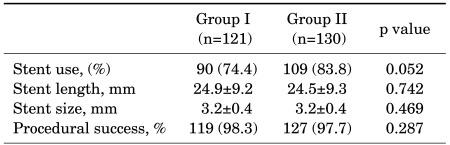
The number of involved vessels was significantly different between the two groups (p=0.006). Group I had a greater proportion of patients with only one vessel involved, whereas Group II had a greater proportion of patients with more than two vessels involved. Proportions of total occlusion, target lesion, and left main stenosis were similar between the two groups. The severity of coronary lesions according to the American College of Cardiology/American Heart Association (ACC/AHA) classification and TIMI flow were not significantly different between the two groups (Table 3). The stent diameter was 3.2±0.4 mm in both groups. Stent length was 24.9±9.2 mm in Group I and 24.5±9.3 mm in Group II (p=0.742). Stent use and procedural success were similar between the two groups (Table 4).
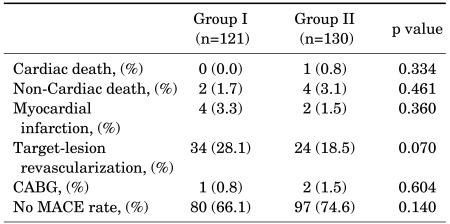
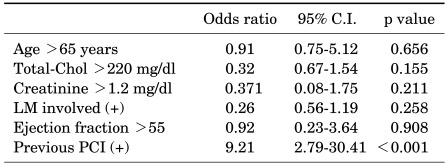
During the mean follow-up period of about 36 months, the rate of MACEs was 33.9% in the group exposed to TCDD and 25.4% in the group not exposed to TCDD, respectively, although the difference was not statistically significant (Table 5). By binary logistic regression analysis, the only independent risk factor for MACE in veterans exposed to TCDD was a previous history of PCI (OR=9.21, 95% CI: 2.79-30.41, p<0.001) (Table 6).
This study examined the effects of TCDD exposure on cardiovascular disease in veterans who had been exposed to the agent and who underwent PCI due to ACS. The study endpoints showed that there was no significant difference in the rates of MACE after intervention between the two groups in this study despite the presence of differences in baseline characteristics. According to these results, we can assume that exposure to TCDD does not affect the severity of cardiovascular disease in acute coronary syndrome.
The results of our study differed somewhat from those of other previous studies. The prevalence of diabetes was significantly higher in the group not exposed to TCDD. However, the prevalence of hypertension and hyperlipidemia, which are well-known risk factors for cardiovascular disease, was significantly higher in the patients who had been exposed to TCDD than in those who had not. A history of previous PCI was significantly higher in Group I than in Group II. Patients in Group I were not concerned about the cost of procedures and hospital admission because Vietnam veterans can use the Veterans Hospital without extra cost in Korea, and they can undergo PCI without hesitation. The proportion of patients with two or more involved vessels was higher in Group II. We presume that patients in Group I readily underwent PCI despite presumably lesser cardiac symptoms because they were not concerned about cost.
Agent Orange was given its name from the color of the 55-gallon orange-striped barrels in which it was shipped. It is a roughly 1:1 mixture of two phenoxyl herbicides in iso-octyl ester form: 2,4-dichlorophenoxyacetic acid (2,4-D) and 2,4,5-trichlorophenoxyacetic acid (2,4,5-T). It was known to contain TCDD, a byproduct of the manufacture of 2,4,5-T.5 Many scientific studies have found sufficient evidence to support a positive association between certain types of cancer and exposure to Agent Orange and its dioxin contaminant. These cancers include soft tissue sarcoma, non-Hodgkin lymphoma, Hodgkin disease, respiratory cancers (lung, larynx, and trachea), prostate cancer, and multiple myeloma.6 Its untoward effects also include reproductive toxicity, teratogenesis, immune dysfunction, and alterations in thyroid hormone mechanisms and body and organ weights.7
Several epidemiological studies have shown a weakly positive association between accidental or occupational exposure to TCDD and glucose metabolism disorders, including insulin resistance, hyperinsulinemia, and type II diabetes mellitus.8,9 Rene et al. proposed a biologically plausible mechanism for this association based on opposing biochemical actions of the activated aryl hydrocarbon receptor (AhR) and peroxisome proliferator activated receptors (PPARs). Whereas activated PPARs promote the differentiation of adipocytes, which mediate glucose and lipid homeostasis and the expression of the glucose transporter protein GLUT4, dioxin-like compounds inhibit preadipocyte differentiation and reduce glucose transport activity and the copy number of both GLUT4 and its mRNA. Rene and his colleagues proposed that these mechanisms induce diabetes mellitus.10,11
There are several animal studies on the association between exposure to TCDD and the cardiovascular system. David et al. examined the cardiac effects of TCDD by using atrial muscle isolated from young or mature guinea pigs. Atrial muscle from TCDD-treated guinea pigs had a significantly lower basal force of contraction after 20 days of treatment. They concluded that TCDD adversely affects the atrial muscle of the guinea pig heart.12 On the other hand, some reports suggested that there were no direct effects of TCDD exposure on the cardiovascular system. By assessing the ratio of heart ventricular mass to body weight and the activities of pyruvate kinase and citrate synthase in homogenates of heart ventricular muscle, Christopher et al. proposed that overtly toxic doses of TCDD in the rat did not depress mechanical function of the heart.13 Hermansky et al. showed that the effects of TCDD on cardiac function did not appear to be due to a direct action of the xenobiotic on the heart but possibly to down-regulation of β-receptors in the heart as a result of the hypothyroid state.14,15
In some epidemiologic studies, Vietnam veterans had an increased frequency of cardiovascular disease. Kim et al. found a link between Agent Orange and high blood pressure. Risk of high blood pressure can be very serious and can adds other cardiac problems, such as ischemic heart disease and valvular heart disease, to the growing list of health concerns for veterans of the Vietnam War.16 Kang et al. suggested that Vietnam veterans who were occupationally exposed to Agent Orange experienced a higher risk of several chronic medical conditions than did other non-Vietnam veterans.17
This study had a few limitations. First, we supposed that all Vietnam War veterans had been exposed to Agent Orange. However, this may not have been true. Also, we could not accurately determine the degree or length of exposure to Agent Orange. Because of this selection bias, this study had limited power to evaluate the effect of TCDD on the outcome of ACS. Secondly, inferences were limited by the relatively small size of the population studied (N=251). All veterans in this study were older men, and the effects of TCDD on the hearts of women and younger individuals may differ. To address these limitations and to more accurately determine the effects of TCDD, a longitudinal and large-scale investigation is needed.
In conclusion, despite the higher incidence of hypertension and hyperlipidemia in patients exposed to TCDD, exposure to TCDD may not affect the severity of cardiovascular disease and the rate of MACEs in ACS.
References
1. Gaylor DW, Aylward LL. An evaluation of benchmark dose methodology for non-cancer continuous-data health effects in animals due to exposures to dioxin (TCDD). Regul Toxicol Pharmacol. 2004; 40:9–17. PMID: 15265602.
2. Abbott BD. Review of the interaction between TCDD and glucocorticoids in embryonic palate. Toxicology. 1995; 105:365–373. PMID: 8571373.
3. Tuomisto JT, Pohjanvirta R, Unkila M, Tuomisto J. TCDD-induced anorexia and wasting syndrome in rats: effects of diet-induced obesity and nutrition. Pharmacol Biochem Behav. 1999; 62:735–742. PMID: 10208380.
4. Mehta SR, Granger CB, Boden WE, Steg PG, Bassand JP, Faxon DP, et al. TIMACS Investigators. Early versus delayed invasive intervention in acute coronary syndromes. N Engl J Med. 2009; 360:2165–2175. PMID: 19458363.
5. Harrison RJ. Chemicals and gases. Prim Care. 2000; 27:917–982. PMID: 11072294.
6. Institute of Medicine (U.S.). Committee to Review the Health Effects in Vietnam Veterans of Exposure to herbicides. Veterans and Agent Orange : Health effects of herbicides used in Vietnam. 1994. Washington D.C.: National academy press;p. 433–452.
7. Pavuk M, Schecter AJ, Akhtar FZ, Michalek JE. Serum 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) levels and thyroid function in Air Force veterans of the Vietnam War. Ann Epidemiol. 2003; 13:335–343. PMID: 12821272.
8. Henriksen GL, Ketchum NS, Michalek JE, Swaby JA. Serum dioxin and diabetes mellitus in veterans of Operation Ranch Hand. Epidemiology. 1997; 8:252–258. PMID: 9115019.
9. Calvert GM, Sweeney MH, Deddens J, Wall DK. Evaluation of diabetes mellitus, serum glucose, and thyroid function among United States workers exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Occup Environ Med. 1999; 56:270–276. PMID: 10450245.
10. Remillard RB, Bunce NJ. Use of Haber's Rule to estimate the risk of diabetes from background exposures to dioxin-like compounds. Toxicol Lett. 2002; 131:161–166. PMID: 11992735.
11. Ray S, Swanson HI. Activation of the aryl hydrocarbon receptor by TCDD inhibits senescence: a tumor promoting event? Biochem Pharmacol. 2009; 77:681–688. PMID: 19100242.
12. Brewster DW, Matsumura F, Akera T. Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on guinea pig heart muscle. Toxicol Appl Pharmacol. 1987; 89:408–417. PMID: 3603569.
13. Kelling CK, Menahan LA, Peterson RE. Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin treatment on mechanical function of the rat heart. Toxicol Appl Pharmacol. 1987; 91:497–501. PMID: 3424379.
14. Hermansky SJ, Holcslaw TL, Murray WJ, Markin RS, Stohs SJ. Biochemical and functional effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on the heart of female rats. Toxicol Appl Pharmacol. 1988; 95:175–184. PMID: 3420610.
15. Kanzawa N, Kondo M, Okushima T, Yamaguchi M, Temmei Y, Honda M, et al. Biochemical and molecular biological analysis of different responses to 2,3,7,8-tetrachlorodibenzo-p-dioxin in chick embryo heart and liver. Arch Biochem Biophys. 2004; 427:58–67. PMID: 15178488.
16. Kim JS, Lim HS, Cho SI, Cheong HK, Lim MK. Impact of Agent Orange exposure among Korean Vietnam veterans. Ind Health. 2003; 41:149–157. PMID: 12916744.
17. Kang HK, Dalager NA, Needham LL, Patterson DG Jr, Lees PS, Yates K, et al. Health status of Army Chemical Corps Vietnam veterans who sprayed defoliant in Vietnam. Am J Ind Med. 2006; 49:875–884. PMID: 17006952.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download