Abstract
Arcanobacterium haemolyticum was isolated from the blood cultures of a previously healthy 37-year-old man who met all the criteria of Lemierre syndrome, including a primary oropharyngeal infection, evidence of thrombophlebitis of the internal jugular vein, and metastatic infections. To the best of our knowledge, this is the first case of Lemierre syndrome caused by A. haemolyticum in Korea and shows that A. haemolyticum alone can cause Lemierre syndrome.
Lemierre syndrome suggested by Sinave et al.1 includes 1) a primary oropharyngeal infection, 2) positive blood culture, 3) evidence of thrombophlebitis of the internal jugular vein (IJV), and 4) metastatic infections. The pathogenesis of Lemierre syndrome involves the IJV by direct extension through the fascial plane between the tonsils and the parapharyngeal space or by a hematogenous or lymphatic spread from a peritonsillar vessel. Once thrombophlebitis of the IJV occurs, septic emboli can arise and spread to distant sites and organs. More than 80% of these cases are related to an underlying Fusobacterium necrophorum infection.2 In 15% of cases, polymicrobial infections with other oral flora such as Peptostreptococcus, Streptococcus, and Bacteroides species have been described.1 Until now, Lemierre syndrome caused by Arcanobacterium haemolyticum, another oral microbe, has been very rare. Only one case of Lemierre syndrome has been reported with blood cultures that were positive for A. haemolyticum as a single pathogen.3
A. haemolyticum is a pleomorphic, gram-positive, non-spore-forming facultative anaerobe. It is frequently overlooked or misidentified as a contaminant or normal flora in the clinical setting because its growth is usually inhibited by other members of the flora, it slowly develops hemolysis in sheep blood agar, and its colony morphology resembles beta-hemolytic streptococci.4 However A. haemolyticum has been isolated from systemic and deep-seated human infections.5 So, clearly, recognition of the ability of this organism to cause disease is important in order to make a correct diagnosis, to understand the pathogenesis, and to commence appropriate antibiotic therapy.
Here we present a case of Lemierre syndrome caused by A. hemolyticum, which was identified by 16s rRNA sequencing and a reverse CAMP test. To our knowledge, this is the first case of Lemierre syndrome caused by A. haemolyticum in Korea. Our case suggests that F. necrophorum is not essential for development of Lemierre syndrome.
A 37-year-old, previously healthy man came into the emergency department of Chonnam National University Hospital with a 3-day history of painful left neck swelling, myalgia, asthenia, and fever. The patient also complained of lower back pain, chest tightness, dyspnea, and cough. He had been treated with cephradine YuHan (YuHan Corporation, Korea, 500 mg three times per day, orally, for 3 days) owing to complaints of a sore throat at another primary clinic. He had no history of intravenous drug abuse and no risk factors for HIV infection. On physical examination, he had a temperature of 38.9℃, a respiratory rate of 28 breaths/min, a pulse of 100 beats/min, and blood pressure of 80/40 mmHg. His throat examination showed mild erythema. The anterior border of the left sternocleidomastoid muscle was very tender on palpation. Breath sounds were clear and there was no heart murmur. A maculopapular rash was observed on the trunk. The results of laboratory tests on admission were as follows: leukocyte count, 2,500 (4,800-10,800 cells/µl) with 79.2% neutrophils; hemoglobin, 13.0 (12-18 g/dl); hematocrit, 36 (37-52%); platelet count, 22×103 (130-450×103/µl) without morphologic changes; alanine aminotransferase, 65 (<37 U/L); aspartate aminotransferase, 38 (<37 U/L); lactate dehydrogenase, 486 (<37 U/L); total bilirubin, 3.98 (<0.6 mg/dl); total protein, 5.2 (6-8.3 g/dl); albumin, 2.6 (3.5-5 g/dl); ESR, 40 (<20 mm/h); and CRP, 20.2 (<0.5 mg/dl). His arterial oxygen pressure (PaO2) was 84.6 mmHg. The serology results were negative for Mycoplasma pneumoniae, Chlamydia pneumoniae, human immunodeficiency virus, hepatitis B virus, and hepatitis C virus. Two sets of aerobic and anaerobic blood culture samples were taken from two different sites. The chest radiography revealed a multiple patchy infiltration and a contrast-enhanced computed tomography scan demonstrated multiple pulmonary consolidation with some internal cavitations predominantly in the subpleural, lower, and peripheral lung. A transthoracic echocardiogram did not show any valvular lesions. Empiric treatment was started with ceftriaxone (Boryung Pharm Co. Ltd, Korea) 2 g every 24 hours and metronidazole (JW Pharmaceutical, Korea) 500 mg every 8 hours.
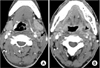
On the third day, the patient was still febrile and the signs of sepsis persisted. A computerized tomographic angiogram (CTA) of the neck was performed, showing an ill-defined non-enhancing filling defect in the left IJV consistent with thrombophlebitis with a 1.9×1.1 cm sized peritonsillar abscess (Fig. 1).
On the seventh day, his condition deteriorated, and his pulmonary symptoms worsened. A chest X-ray was done, showing increased infiltration on both lower lung fields and the development of pleural effusion. A diagnostic thoracentesis was done that resulted in exudate. However, no organisms were cultured from the pleural fluid.
Gram-positive bacilli were found in two sets of blood cultures obtained initially and were identified as A. haemolyticum by use of a VITEK II automated system (bioMérieux, Marcy l'Etoile, France). The isolate was nonmotile, non-acid-fast, catalase-negative, oxidase-negative, and urease-negative. A reverse CAMP test showed a positive result. 16S ribosomal RNA gene sequencing was used to confirm the result. The 5'-AGTTTGATCCTGGCTCAGGTATTGCCGCCGCTGCTG-3' primer was used to amplify the 16S rRNA gene. We found 99% homology in 157 bp of 16S rRNA gene sequences of the isolates and A. haemolyticum (Gen-Bank accession number CP002045.1). Although there is no standardized method for studying the antibiotic susceptibility of A. haemolyticum, the diffusion disc-plate method was performed in this case.3 Susceptibility was approximated by using the zone diameter interpretive standards for Streptococcus spp. established by the Clinical and Laboratory Standard Institute (Performance Standards for Antimicrobial Susceptibility Testing; Twenty-first Information supplement M100-S21). The isolates showed susceptibility only to ciprofloxacin, gentamicin, vancomycin, and chloramphenicol but resistance to penicillin, erythromycin, clindamycin, tetracycline, and trimethoprim/sulfamethoxazole.
Antibiotics were changed to intravenous vancomycin 1 g every 12 hours and gentamycin 100 mg every 8 hours. Simultaneous anticoagulation therapy was started with intravenous heparin.
After that, the patient became afebrile and the other signs of sepsis improved. After 4 weeks of antibiotics therapy, a follow-up CTA of the neck was performed, which and showed the disappearance of the peritonsillar abscess and left IJV thrombosis with improvement of perivascular inflammatory change.
Lemierre syndrome was a very common disease with a high mortality rate in the pre-antibiotic era, but after the introduction of antibiotics, its incidence decreased. However, a recent systematic review reported that Lemierre syndrome with an associated mortality rate of 5% to 22% may not be as rare as previously thought, and that this apparent increase in incidence may be due to antibiotic resistance or changes in antibiotic prescription patterns.6
To the best of our knowledge, only three cases of Lemierre syndrome caused by A. haemolyticum (1 case of single organism and 2 cases of co-infection) have been reported in the medical literature.3 The case that had a single pathogen3 was a healthy man similar to our patient except that he had contact with animals before the onset of his symptoms. Our patient had a characteristic maculopapular rash, which is present in 20% to 25% of A. haemolyticum pharyngitis patients. The two cases including ours revealed that the presence of F. necrophorum is not essential for the development of Lemierre syndrome, and that A. haemolyticum must be considered a potential pathogen in immunocompetent patients. Our case expands the spectrum of disease of A. haemolyticum to include typical Lemierre syndrome.
It is known that A. haemolyticum can be found on the skin and in the pharynx of healthy humans. It has been found in symptomatic individuals either as a sole pathogen or as a component of polymicrobial infection.5 Infections caused by A. haemolyticum, particularly in the upper respiratory tract, are likely underreported. A previous report estimated that approximately 0.5% to 2.5% of all cases of pharyngitis are caused by A. haemolyticum.4 It is presumed that Lemierre syndrome caused by A. haemolyticum occurs more frequently than is reported.
For the successful management of Lemierre syndrome, aggressive intravenous antibiotic therapy based on a microbiological result (if available) is essential, as well as surgical management and anticoagulation therapy. Identifying the causative organism is not always possible. Therefore, understanding possible causative organisms and antibiotic susceptibility is important for appropriate empirical antibiotic therapy. A. haemolyticum produces an uncharacterized hemolytic agent and two biochemically defined extracellular products: a neuraminidase and a phospholipase D (PLD) that act preferentially on sphingomyelin and generate ceramide phosphate in the target membrane. Of these, PLD is known to bring about tissue damage and is thought to participate in vascular permeability and dissemination of the pathogen.7 This partly explains why A. haemolyticum can be sufficiently virulent and render invasive diseases such as Lemierre syndrome.
There are no established guidelines for the treatment of A. haemolyticum, although A. haemolyticum is susceptible to most classes of antimicrobials, including penicillins, cephalosporins, carbapenems, macrolides, fluoroquinolones, tetracyclines, rifampin, and vancomycin.8 High doses of penicillin, with or without gentamicin, are recommended for the treatment of deep infections.5 Although A. haemolyticum is sensitive to penicillin according to in vitro minimal inhibitory concentration (MIC) determinations, failure to obtain a bacteriological or clinical cure following penicillin treatment has been reported.9 Osterlund proposed that the mechanism of penicillin treatment failure could be the survival of intracellularly residing bacteria.9 Because of the possibility of treatment failure, the validity of the antimicrobial susceptibility test of A. haemolyticum should be reevaluated and the MIC results obtained by any method should be interpreted with caution. Macrolides (such as erythromycin and azithromycin) are a reasonable second-line option. However, these agents are bacteriostatic and distribute extensively into the tissues, which may limit their effectiveness in bacteremia cases.10 In our case, the strain was sensitive to vancomycin, gentamicin, ciprofloxacin, and chloramphenicol and was resistant to penicillin, erythromycin, clindamycin, and tetracycline. We did not test cephalosporin. We switched the antibiotics to vancomycin and gentamycin from ceftriaxone and metronidazole after we obtained the results of the susceptibility test. After that, the follow up x-ray result was nearly normalized and the patient's condition improved. Vancomycin and gentamicin combination therapy must be considered if an organism is resistant or upon the failure of first-line therapy.
In our patient, the A. haemolyticum was resistant to penicillin, erythromycin, clindamycin, and tetracycline by the in vitro disc-diffusion method and clinically to 3rd generation cephalosporin. Because Lemierre syndrome is related to upper respiratory tract infection, the poor activities of antibiotics frequently prescribed for upper respiratory tract infection may cause great concern. Although antibiotic resistance is not frequently reported in clinical isolates, considering the trend of increasing antimicrobial resistance to gram-positive bacilli and changes in antibiotic prescription patterns, the emergence of resistant A. haemolyticum is a possibility.
In conclusion, we report the first case of Lemierre syndrome with A. haemolyticum in Korea. A. haemolyticum alone can cause Lemierre syndrome.
Figures and Tables
References
1. Sinave CP, Hardy GJ, Fardy PW. The Lemierre syndrome: suppurative thrombophlebitis of the internal jugular vein secondary to oropharyngeal infection. Medicine (Baltimore). 1989. 68:85–94.

2. Hagelskjaer Kristensen L, Prag J. Human necrobacillosis, with emphasis on Lemierre's syndrome. Clin Infect Dis. 2000. 31:524–532.

3. Fernández-Suárez A, Benítez JM, Vidal AM, Iglesias JM. Lemierre's syndrome and septicaemia caused solely by Arcanobacterium haemolyticum in a young immunocompetent patient. J Med Microbiol. 2009. 58:1645–1648.
4. Linder R. Rhodococcus equi and Arcanobacterium haemolyticum: two "coryneform" bacteria increasingly recognized as agents of human infection. Emerg Infect Dis. 1997. 3:145–153.

5. Skov RL, Sanden AK, Danchell VH, Robertsen K, Ejlertsen T. Systemic and deep-seated infections caused by Arcanobacterium haemolyticum. Eur J Clin Microbiol Infect Dis. 1998. 17:578–582.

6. Karkos PD, Asrani S, Karkos CD, Leong SC, Theochari EG, Alexopoulou TD, et al. Lemierre's syndrome: A systematic review. Laryngoscope. 2009. 119:1552–1559.

7. Lucas EA, Billington SJ, Carlson P, McGee DJ, Jost BH. Phospholipase D promotes Arcanobacterium haemolyticum adhesion via lipid raft remodeling and host cell death following bacterial invasion. BMC Microbiol. 2010. 10:270.
8. Therriault BL, Daniels LM, Carter YL, Raasch RH. Severe sepsis caused by Arcanobacterium haemolyticum: a case report and review of the literature. Ann Pharmacother. 2008. 42:1697–1702.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download