INTRODUCTION
Coronary artery ectasia (CAE) is the localized or diffuse dilatation of the coronary artery with a diameter at least 1.5 times the adjacent normal coronary artery. The incidence of CAE is reported to be 0.3% to 4.9% in patients undergoing diagnostic coronary angiography (CAG) or as determined by autopsy.1,2 CAE can be asymptomatic; however, sometimes patients present with exertional chest pain, acute coronary syndrome, arrhythmia, and sudden death. Complications such as thrombus, distal embolization, and shunt formation can occur.3 Currently, there is no evidence-based guideline for stenotic ectatic coronary arteries because of the various sizes and types of ectatic coronary arteries. We report here two cases of stenotic CAE that were successfully treated with different angioplasty modalities.
CASE REPORT
1. Case 1: balloon angioplasty alone
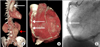
A 72-year-old man presented with exertional chest pain. He was an ex-smoker and had diabetes mellitus. He visited the department of chest surgery for a palpable abdominal mass. The diagnostic work-up showed ascending and abdominal aortic aneurysm, aortic stenosis, and severe discrete concentric stenosis in the mid portion of the right coronary artery (RCA) with mild ectatic change (Fig. 1). Aortic valve replacement (AVR; with a Carpentier-Edwards Perimount Magna 23-mm valve), reduction aortoplasty of the ascending aorta (with the suture plication technique and external wrapping with a 26-mm vascular graft), and coronary artery bypass graft (CABG; left great saphenous vein from aorta to distal RCA) was done. After 6 months, replacement of the abdominal aorta with a Hemashield 20-mm straight graft was done.
At 7 months after the last surgery, the patient developed exertional chest pain. Coronary computed tomography (CT) angiography showed two focal sites of significant, eccentric stenosis in the mid RCA and total occlusion of the LGSV to RCA. (Fig. 2). Echocardiography showed acceptable bioprosthetic aortic valve function and no regional wall motion abnormalities.
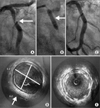
CAG through the right femoral artery was performed. The angiographic findings showed moderate tubular concentric stenosis in the proximal RCA and severe discrete concentric stenosis in the mid RCA with ectatic changes. However, blood flow from the LGSV to RCA was not shown, which suggested total occlusion (Fig. 3A).
Because of the larger size of the stenotic CAE, balloon angioplasty with parallel stenting was considered. However, balloon angioplasty alone without stenting was planned initially because of the possibility of early discontinuation of dual anti-platelet agents because of the chance of additional surgery for underlying valvular heart disease and aortic disease. The patient had previously undergone major cardiac surgery twice before owing to aortic stenosis and aortic aneurysm. Although the prosthetic aortic valve was intact, the aortic aneurysm was repaired through a staged operation and the graft did not entirely cover the aneurysm. Thus, we considered the possibility of future additional operative repair.
With a 7F AR2 catheter as a guide (Medtronic Inc., Minneapolis, MN,USA), balloon dilation was done with an Avita 3.0×15-mm balloon (Orbusneich, Wanchai, Hong Kong) and residual stenosis was more than 70%. Further ballooning was done with a Mercury 4.5×20-mm balloon (Abbott Vascular, Illinois, USA) and residual stenosis was less than 30% (Fig. 3B).
The patient was on the dual antiplatelet agents (aspirin and clopidogrel), a lipid-lowering agent (simvastatin), a calcium channel blocker (diltiazem), and a beta-blocker (carvedilol) after the percutaneous coronary intervention. He was safely discharged without chest pain or any other cardiovascular events. Routine follow-up CAG at 6 months showed no significant restenosis at the previous balloon angioplasty site (Fig. 3C). Dual antiplatelet agents were maintained for 2 years because of his history of diabetes and restenosis after CABG.
2. Case 2: peripheral balloon and stent
A 46-year-old man presented with resting chest pain. He had suffered from hypertension and dyslipidemia for 5 years and had a history of smoking. He had no significant findings on the electrocardiogram, in the echocardiography, or for cardiac enzymes.
CAG via the right radial artery was performed. The angiographic findings showed severe tubular eccentric stenosis in the mid portion of the left circumflex artery (LCX) with ectatic change in both the left anterior descending artery (LAD) and LCX (Fig. 4A). The RCA was relatively hypoplastic.
Elective percutaneous coronary angioplasty was planned. Because of ectasia in the target lesion as shown by the angiographic and intravascular ultrasound (IVUS) measurements (Fig. 4D), we could not find a proper device size among the usual coronary balloons and stents. We decided to use a larger device commonly used in the peripheral artery.
Right femoral artery puncture and guiding was performed with a 7F EBU 3.5 guiding catheter (Medtronic Inc., Minneapolis, MN, USA). Before percutaneous coronary angioplasty, the IVUS exam showed significant, eccentric plaque burden from the 5 o'clock to the 7 o'clock direction and the reference vessel diameter measured just distal to the target lesion was 6.2×6.8 mm (Fig. 4D). In the mid LCX lesion, predilation was done with an Amiia PTA balloon catheter measuring 5.0×20 mm (Cordis Europa, N.V., Roden, Netherlands). After predilation, residual stenosis was approximately 55% to 60% with mild intimal dissection. A Genesis 6.0×24-mm stent (Palmaz® Genesis™, Cordis Europa, N.V.) was successfully deployed in the mid LCX lesion, and subsequent adjuvant ballooning was done for angiographic optimal stent expansion with an Amiia 7.0×20-mm balloon catheter (Cordis Europa, N.V.; Fig. 4C). Final coronary angiography and IVUS showed a well expanded and opposed Genesis stent (Fig. 4E).
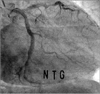
The patient no longer had chest pain and was safely discharged for clinical follow-up. He was placed on dual antiplatelet agents (aspirin and clopidogrel), a lipid-lowering agent (rosuvastatin), a calcium channel blocker (diltiazem), and trimetazidine. A routine follow-up CAG at 6 months showed patent previous stent in the mid LCX without in-stent restenosis (Fig. 5). After the follow-up CAG, aspirin monotherapy was maintained because the implanted peripheral stent was a bare metal stent.
DISCUSSION
Treatment of CAE includes medical management, stent implantation, and surgical excision. The medical management of CAE includes antiplatelets, anticoagulation, anti-spasm therapy (calcium channel blocker and nitrate), ACE inhibitors, and trimetazidine.3,4 Stent implantation is recommended when significant stenosis accompanies the CAE and medical therapy has not been successful.5,6 If the target lesion is not suitable for stent implantation, surgical excision or ligation with a bypass graft could be a choice for treatment.7
Especially in stenotic ectatic coronary arteries, stent implantation is considered for patients with refractory angina despite medical management; however, because of the heterogeneity of the coronary artery morphology and relatively larger vessel size, it is difficult to determine stent type, size, and complete expansion with the usual coronary devices. Thus, there have been limited data regarding revascularization of stenotic ectatic coronary arteries and no current guidelines for selecting a specific stent.
Because of the variable size and morphology of arteries with CAE, various stents have been utilized for revascularization. Agirbasli et al. reported 3 cases of coronary angioplasty with intra-therapeutic peripheral biliary stents for CAE.8 This was the first report of using a peripheral biliary stent in a coronary vascular structure. Stefanadis et al. reported the successful deployment of an autologous venous graft-covered stent to a coronary aneurysm.9 Rha et al. reported a challenging case of "parallel stenting" in which two 3.5×180-mm sirolimus-eluting stents were used in parallel to each other in a patient with CAE with IVUS guidance.10 In a literature review of the management of CAE cases, we found no standard treatment option or long-term follow-up results with revascularization therapy. In previous cases, the use of an intrahepatic peripheral biliary stent and a venous graft-covered stent were reported. However, these options are not common and are not familiar to coronary interventionists. Our treatment modality with a peripheral artery balloon and stent may be a more proper option for the coronary interventionist because of experience, accessibility, and familiarity. In this aspect, our cases could suggest a new treatment modality using peripheral devices for the management of huge coronary ectasia.
In our first case, stenosis in the native RCA with CAE was aggravated owing to total occlusion of the bypass graft (LGSV to RCA flow). Because of serious comorbidities and the possibility of a future surgical approach, only balloon angioplasty was done. If the follow-up CAG showed significant re-stenosis at the balloon angioplasty site, we were considering "parallel stenting" using two coronary drug-eluting stents or "parallel ballooning" using two drug-eluting balloons. However, the follow-up CAG at 6 months showed no significant stenosis in the balloon angioplasty site. Because of the relatively larger reference vessel size of the CAE patient, recurrence causing real ischemia by functional study using a pressure wire would be less even if the visual angiographic restenosis appeared to be significant compared with recurrence in the usual native coronary arteries. This case suggests that balloon angioplasty alone can be a good treatment option in CAE patients with significant stenosis.
In our second case, we utilized a peripheral artery balloon and deployed a peripheral artery stent in the stenotic lesion of the ectatic coronary artery. Although there were some resistance and friction during device positioning owing to the relatively larger profile compared with the usual coronary device, there was no immediate mechanical complications or subsequent cardiovascular events with the balloon and stent designed for peripheral artery disease. To the best of our knowledge, this is the first report of peripheral artery stenting in a patient with CAE.
Our cases suggest other specific revascularization options for stenosis in patients with CAE. (1) In selected patients, balloon angioplasty alone can produce acceptable results without significant recurrence or cardiovascular events during up to 1 year of clinical follow-up. (2) In selected patients, a peripheral artery balloon and stent Could be safely adopted with acceptable results in patients with CAE with a stenotic lesion.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download