Abstract
Daily use of probiotic chewing gum might have a beneficial effect on oral health, and it is important that the viability of the probiotics be maintained in this food product. In this study, we examined the stability of probiotic chewing gum containing Weissella cibaria. We evaluated the effects of various factors, including temperature and additives, on the survival of freeze-dried probiotic W. cibaria powder. No changes in viability were detected during storage at 4℃ for 5 months, whereas the viability of bacteria stored at 20℃ decreased. The stability of probiotic chewing gum decreased steadily during storage at 20℃ for 4 weeks. The viability of the freeze-dried W. cibaria mixed with various additives, such as xylitol, sorbitol, menthol, sugar ester, magnesium stearate, and vitamin C, was determined over a 4-week storage period at 20℃. Most of the freeze-dried bacteria except for those mixed with menthol and vitamin C were generally stable during a 3-week storage period. Overall, our study showed that W. cibaria was more stable at 4℃ than that at 20℃. In addition, menthol and vitamin C had a detrimental effect on the storage stability of W. cibaria. This is the first study to examine the effects of various chewing gum additives on the stability of W. cibaria. Further studies will be needed to improve the stability of probiotic bacteria for developing a novel probiotic W. cibaria gum.
The food industry often refers to foods supplemented with probiotics as functional foods. Several health benefits are associated with the consumption of live probiotic bacteria.1 Because of the therapeutic benefits of live probiotics, there has been an increase in the incorporation of probiotic bacteria in a wider variety of food products, including yogurts, cheese, drinks, chewing gum, and dietary supplements.2,3 Probiotics are live microorganisms that can confer a health benefit to the host when consumed in adequate amounts.4 Interest in the use of foods that contain probiotics is increasing, and various functional foods that contain these microorganisms have been developed. The viability of the probiotics must be maintained in the food product until the time of consumption, and the probiotics should be present in significant numbers, at least 107 viable cells per gram or milliliter of a product.4 The survival of probiotics is genus-, species-, and strain-dependent, and the composition of the other ingredients in the preparation will also affect viability.5 For this reason, changes in the number of viable probiotic bacteria during storage of functional foods should be studied more extensively.
Weissella species are lactic acid bacteria that were formerly included in the genus Lactobacillus.6 Weissella cibaria is a Gram-positive, non-spore-forming, nonmotile, heterofermentative, catalase-negative bacillus that is normally isolated from fermented foods. Recently, our research group showed that W. cibaria isolates from human saliva can be used as a probiotic owing to an inhibitory effect on biofilm formation and volatile sulfur compound formation.7,8 Previous studies have demonstrated that daily use of probiotic chewing gum has a beneficial effect on oral health.9,10 Hence, the goal of this study was to investigate the stability of probiotic chewing gum containing W. cibaria.
For the development of functional foods, including probiotic chewing gum, probiotics should have the ability to remain viable and to retain their probiotic properties during production and storage of the probiotic food product.4 Commercially available chewing gums are often supplemented with various ingredients, such as sugars, vitamins, and various flavors in various amounts. All these ingredients will have an effect on the growth and activity of probiotic bacteria. To avoid such variability, it is important to examine the stability of probiotic bacteria in a complex mixture. Therefore, the objective of this study was to examine the effect of temperature on the storage stability of freeze-dried probiotic W. cibaria as well as the influence of various additives on the viability of W. cibaria in a mixture. This study aimed to gain a better understanding of the role of these ingredients on probiotic survival in order to develop a novel probiotic gum.
The strain W. cibaria CMU, which has been shown to possess probiotic properties,8 was used as a model microorganism.
Freeze-dried W. cibaria powders were produced and provided by CTC BIO (Seoul, South Korea). The powders were stored in sterile plastic tubes at 4℃ or 20℃ for 5 months. The viable counts of the stored powders were determined after 0, 1, 2, 3, 4, and 5 months of storage. To count the viable cells of W. cibaria, 0.1 g of lyophilized powder was homogenized in 1 ml of phosphate-buffered saline. Serial dilutions were spread onto De Man, Rogosa, Sharpe agar plates (MRS agar; Difco, Detroit, MI, USA), which were then incubated aerobically at 37℃ for 24 to 48 hours.
The probiotic chewing gums were manufactured and provided by Xybita (Seoul, South Korea). The gums contained 2% freeze-dried W. cibaria powder, which corresponded to 1.7 g per tablet. The concentration of W. cibaria in the gum was 8.8×109 colony-forming units (CFU) per gum. The gum also contained xylitol, sorbitol, menthol, sugar ester, magnesium stearate, vitamin C, glycerin, and flavor. The pieces of gum were stored in sterile plastic tubes at 20℃ for 4 weeks. One tablet of chewing gum was aseptically taken for microbial analysis at weekly intervals. To investigate the viability of W. cibaria, the probiotic chewing gum tablet was ground and homogenized in 10 ml of phosphate-buffered saline for 20 min. Serial dilutions were spread onto MRS plates, which were incubated aerobically at 37℃ for 24 to 48 hours.
To evaluate the effects of the various additives used in chewing gum on the viability of W. cibaria, equal amounts of xylitol, sorbitol, menthol, sugar ester, magnesium stearate, and vitamin C, respectively, were mixed with W. cibaria powder. W. cibaria powder was used as a control. Control and mixed samples were stored at 20℃ for 4 weeks. Samples of approximately 0.1 g were aseptically taken for microbial analysis at weekly intervals. The viability was assessed by bacterial counts, which were performed on MRS plates as described above.
Results are expressed as means±standard deviations of three independent experiments. Statistical analysis was carried out by using SPSS (Statistical Packages for Social Science version 17.0; SPSS Inc., Chicago, IL, USA). A Mann-Whitney U test was used to identify any statistically significant differences in the experiments.
The viability of freeze-dried W. cibaria stored at 4℃ or 20℃ for 5 months is shown in Fig. 1. Both conditions showed an average initial viable count of 1.08×1011 CFU/ml. After 2 months, the survival rates for W. cibaria at 20℃ did not differ significantly from those at 4℃. However, a steady loss in viability was detected, and the viability of W. cibaria stored at 20℃ was approximately 50.0±8.3% of the viability after a storage period of 3 months. After a 4-month storage period, a significantly higher viable population was observed in the dried samples incubated at 4℃ than in those stored at 20℃ (p<0.05). The viable count of bacteria at 20℃ decreased to 2.64×108±1.64×108 CFU/ml at the end of the storage period. However, no changes in viability were detected during storage at 4℃.
The stability of probiotic chewing gum was examined during storage at 20℃ for 4 weeks (Fig. 2). The viability of W. cibaria rapidly decreased with storage period. The cell number of W. cibaria was initially higher (8.80×109±1.20×109 CFU/ml) and decreased by 7-log cycles (4.50×107±2.70×107 CFU/ml) at the end of the storage period. The viability was approximately 0.5±0.2% after storage for 4 weeks.
The viable cell counts of the freeze-dried W. cibaria mixed with various additives were determined over a 4-week storage period at 20℃ (Table 1). In addition, viabilities of the freeze-dried W. cibaria mixed with xylitol, sorbitol, menthol, sugar ester, magnesium stearate, and vitamin C were determined compared with control (Fig. 3). In general, the viability of W. cibaria stored with various additives decreased over the 4-week storage period when compared with control. After a 2-week storage period, the viability of bacteria stored with menthol, vitamin C, or sorbitol was approximately 12.0±1.1%, 48.1±3.1%, and 78.8±12.4%, respectively. Freeze-dried bacteria mixed with xylitol or sugar ester were stable during a 3-week storage period. However, a significantly lower viable population was observed in the mixed samples compared with the control at the end of the storage period (p<0.05). No significant changes in the viability of bacteria stored with magnesium stearate were detected during a 4-week storage period.
The product in which probiotics are ingested or delivered in the oral cavity can affect the oral colonization of the probiotic. Hence, because the daily consumption of probiotics might lead to a transient or permanent colonization of these bacteria, consumption of probiotics might have a beneficial effect on the oral cavity. Specifically, the daily use of high levels of probiotic bacteria is required to confer health benefits. For this reason, the viability of probiotics must be maintained in the food product until the time of consumption.4 Therefore, changes in the number of viable probiotic bacteria during the storage of probiotic-containing products should be studied more extensively.11
Freeze-drying has traditionally been used for preserving the starter culture and probiotic bacteria.12,13 This is because the low temperatures used during freeze-drying are expected to be less injurious to labile biological organisms than drying at ambient or higher temperatures.14 Many factors contribute to the stability of freeze-dried probiotic preparations, including the type of probiotic, method of drying, the composition of the matrix, and storage condition.13,15 The stability of freeze-dried cultures is generally higher at low-temperature storage. Storage under nonrefrigerated conditions at high relative humidity and exposure of powders to an oxygen-containing atmosphere can contribute to a decrease in viability of probiotics over time. The current study showed that the survival of W. cibaria at 20℃ decreased as the storage time increased. W. cibaria survival during storage was higher at 4℃ than at 20℃. The results of this study agree with the findings of other similar studies; for example, the survival of freeze-dried lactic acid bacteria was shown to decrease at higher storage temperatures.16
Several authors have suggested that chewing gums containing probiotic Lactobacillus reuteri could have a beneficial effect on oral health.9,10 The results of these studies have shown that chewing gum containing probiotic bacteria reduces the levels of salivary mutans streptococci as well as the levels of pro-inflammatory cytokines in gingival crevicular fluid. In addition, our previous studies on W. cibaria were the first to use a more scientifically based step-by-step approach to identify a probiotic for the treatment or prevention of oral disease.7,8 It can be anticipated that the repeated daily use of probiotic W. cibaria-containing foods, such as chewing gum, over a long period of time will increase the level of probiotic bacteria in the oral cavity.
Commercially available chewing gums contain various sugars, vitamins, and flavors at different concentrations. All of these ingredients can affect the growth and activity of probiotic bacteria. To avoid such variability, it is important to examine the stability of probiotic bacteria in a complex mixture. Menthol is a monocyclic monoterpene that is the main compound in the essential oil of Mentha piperita.17 It is an organic compound that is naturally occurring in mint plants. It is routinely used as a cooling, anesthetic, antipruritic, and antiseptic agent in many pharmaceutical formulations, toothpastes, and gums.18 Tampieri et al.19 suggested that the essential oil of Mentha piperita can inhibit the growth of Staphylococcus species, Escherichia coli, and C. albicans. Similarly, the present study showed that menthol had a detrimental effect on the storage stability of probiotic W. cibaria. Therefore, a possible mechanism for the decrease in storage stability is that menthol inhibits the viability of W. cibaria.
Previous studies have demonstrated that damage to the cell membrane occurs during long-term storage, and some antioxidants have been reported to protect membrane lipids against such damage.20 The addition of antioxidants may increase the storage stability of the dried probiotic bacteria.15,21 Vitamin C or ascorbic acid is a weak sugar acid that is structurally related to glucose. It usually acts as an antioxidant. It typically reacts with oxidants of the reactive oxygen species, such as the hydroxyl radical formed from hydrogen peroxide.22 Several studies have shown that vitamin C can be beneficial to probiotic survival during storage, presumably because it is an oxygen scavenger, thus promoting a more favorable anaerobic environment.20,23 On the other hand, vitamin C has long been known for its antibacterial properties.24 In the present study, vitamin C did not improve the survival of probiotics. We suggest that the viability of W. cibaria might be decreased by the antibacterial properties of vitamin C.
The polyols most frequently used in chewing gum are sorbitol, a hexitol derived from glucose, and xylitol, a pentitol that occurs widely in nature. Xylitol is a natural sugar alcohol that is commonly used as a sweetener in chewing gums and candy.25,26 Sucrose fatty acid esters, which are generally called sugar esters, are nonionic surfactants consisting of sucrose as a hydrophilic group and a fatty acid as the lipophilic group.27 This compound increases chewing gum plasticity, softness, and chewiness and prevents adherence to the teeth during chewing. Magnesium stearate is insoluble in water and has been used as a filling agent in food supplements. In the present study, the viabilities of W. cibaria mixed with polyols, sugar ester, and magnesium stearate were generally stable during the storage period. Among the additives tested, magnesium stearate resulted in the highest survival when mixed with the bacteria.
In this study, the stability of freeze-dried W. cibaria powders at different temperatures were determined. Our findings showed that W. cibaria did not survive well at 20℃. In addition, this is the first study to examine the effects of various gum additives on the stability of W. cibaria. The data indicate that menthol and vitamin C had detrimental effects on the storage stability of W. cibaria. Further studies will be needed to improve the stability of probiotic bacteria for developing a novel probiotic W. cibaria gum.
Figures and Tables
FIG. 1
The viable cells of freeze-dried W. cibaria as a function of storage time at a storage temperature of 4℃ and 20℃. *p<0.05 for 20℃ vs. 4℃. The values are reported as the mean±SD of three independent experiments.

FIG. 2
Storage stability of probiotic W. cibaria chewing gum as a function of storage time at a storage temperature of 20℃. Viable cell numbers of W. cibariain chewing gum containing W. cibaria were examined. *p<0.05, compared with the initial storage time. The values are reported as the mean±SD of three independent experiments.
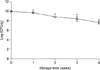
FIG. 3
Comparative effect of additives on the survival rate of freeze-dried W. cibaria as a function of storage time at 20℃. *,†,‡,§,∥p<0.05, compared with control. The values are reported as the mean±SD of three independent experiments.

ACKNOWLEDGEMENTS
This study was supported by a grant (CRI11025-1) of the Chonnam National University Hospital Research Institute of Clinical Medicine.
References
1. Mital BK, Garg SK. Anticarcinogenic, hypocholesterolemic, and antagonistic activities of Lactobacillus acidophilus. Crit Rev Microbiol. 1995. 21:175–214.

2. Phillips M, Kailasapathy K, Tran L. Viability of commercial probiotic cultures (L. acidophilus, Bifidobacterium sp., L. casei, L. paracasei and L. rhamnosus) in cheddar cheese. Int J Food Microbiol. 2006. 108:276–280.

3. Ibrahim F, Ruvio S, Granlund L, Salminen S, Viitanen M, Ouwehand AC. Probiotics and immunosenescence: cheese as a carrier. FEMS Immunol Med Microbiol. 2010. 59:53–59.

4. Kailasapathy K, Chin J. Survival and therapeutic potential of probiotic organisms with reference to Lactobacillus acidophilus and Bifidobacterium spp. Immunol Cell Biol. 2000. 78:80–88.

5. Del Piano M, Morelli L, Strozzi GP, Allesina S, Barba M, Deidda F, et al. Probiotics: from research to consumer. Dig Liver Dis. 2006. 38:Suppl 2. S248–S255.

6. Björkroth KJ, Schillinger U, Geisen R, Weiss N, Hoste B, Holzapfel WH, et al. Taxonomic study of Weissella confusa and description of Weissella cibaria sp. nov., detected in food and clinical samples. Int J Syst Evol Microbiol. 2002. 52:141–148.

7. Kang MS, Chung J, Kim SM, Yang KH, Oh JS. Effect of Weissella cibaria isolates on the formation of Streptococcus mutans biofilm. Caries Res. 2006. 40:418–425.

8. Kang MS, Kim BG, Chung J, Lee HC, Oh JS. Inhibitory effect of Weissella cibaria isolates on the production of volatile sulphur compounds. J Clin Periodontol. 2006. 33:226–232.

9. Caglar E, Kavaloglu SC, Kuscu OO, Sandalli N, Holgerson PL, Twetman S. Effect of chewing gums containing xylitol or probiotic bacteria on salivary mutans streptococci and lactobacilli. Clin Oral Investig. 2007. 11:425–429.

10. Twetman S, Derawi B, Keller M, Ekstrand K, Yucel-Lindberg T, Stecksen-Blicks C. Short-term effect of chewing gums containing probiotic Lactobacillus reuteri on the levels of inflammatory mediators in gingival crevicular fluid. Acta Odontol Scand. 2009. 67:19–24.

11. Vinderola G, Binetti A, Burns P, Reinheimer J. Cell viability and functionality of probiotic bacteria in dairy products. Front Microbiol. 2011. 2:70.

12. Dimitrellou D, Kourkoutas Y, Banat IM, Marchant R, Koutinas AA. Whey-cheese production using freeze-dried kefir culture as a starter. J Appl Microbiol. 2007. 103:1170–1183.

13. Siaterlis A, Deepika G, Charalampopoulos D. Effect of culture medium and cryoprotectants on the growth and survival of probiotic lactobacilli during freeze drying. Lett Appl Microbiol. 2009. 48:295–301.

14. Franks F. Freeze-drying of bioproducts: putting principles into practice. Eur J Pharm Biopharm. 1998. 45:221–229.

15. Wang YC, Yu RC, Chou CC. Viability of lactic acid bacteria and bifidobacteria in fermented soymilk after drying, subsequent rehydration and storage. Int J Food Microbiol. 2004. 93:209–217.

16. Broadbent JR, Lin C. Effect of heat shock or cold shock treatment on the resistance of Lactococcus lactis to freezing and lyophilization. Cryobiology. 1999. 39:88–102.

17. Patel T, Ishiuji Y, Yosipovitch G. Menthol: a refreshing look at this ancient compound. J Am Acad Dermatol. 2007. 57:873–878.

18. Galeotti N, Di Cesare Mannelli L, Mazzanti G, Bartolini A, Ghelardini C. Menthol: a natural analgesic compound. Neurosci Lett. 2002. 322:145–148.

19. Tampieri MP, Galuppi R, Macchioni F, Carelle MS, Falcioni L, Cioni PL, et al. The inhibition of Candida albicans by selected essential oils and their major components. Mycopathologia. 2005. 159:339–345.

20. Carvalho AS, Silva J, Ho P, Teixeira P, Malcata FX, Gibbs P. Effects of various sugars added to growth and drying media upon thermotolerance and survival throughout storage of freeze-dried Lactobacillus delbrueckii ssp. bulgaricus. Biotechnol Prog. 2004. 20:248–254.

21. Kurtmann L, Carlsen CU, Risbo J, Skibsted LH. Storage stability of freeze-dried Lactobacillus acidophilus (La-5) in relation to water activity and presence of oxygen and ascorbate. Cryobiology. 2009. 58:175–180.

23. Shah NP, Ding WK, Fallourd MJ, Leyer G. Improving the stability of probiotic bacteria in model fruit juices using vitamins and antioxidants. J Food Sci. 2010. 75:M278–M282.

24. Myrvik QN, Volk WA. Comparative study of the antibacterial properties of ascorbic acid and reductogenic compounds. J Bacteriol. 1954. 68:622–626.

25. Shaw JH. Inability of low levels of sorbitol and mannitol to support caries activity in rats. J Dent Res. 1976. 55:376–382.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download