Abstract
The present study investigated the changes that occurred in the mammalian target of rapamycin (mTOR) signaling pathway in the kidney as a result of deoxycorticosterone acetate (DOCA)-salt hypertension. Rats were implanted with DOCA strips (200 mg/kg) 1 week after unilateral nephrectomy and were then supplied with 0.9% saline to drink. Four weeks after DOCA implantation, systolic blood pressure (SBP) was measured by use of the tail-cuff method. The expression levels of phosphorylated phosphatidylinositol-3-kinase (PI3K), Akt, and mTOR, as well as the protein expression levels of ED-1 and cyclooxygenase-2 (COX-2), transforming growth factor-β1 (TGF-β1), α-smooth muscle actin (SMA), caspase-3, Bax, and Bcl-2, were then examined in the kidney by semiquantitative immunoblotting. DOCA-salt hypertensive rats were found to have significantly increased SBP as well as an increased kidney weight-to-body weight ratio. Moreover, the phosphorylation of PI3K, Akt, and mTOR was increased in the kidney of DOCA-salt hypertensive rats compared with the control, as was the protein expression of ED-1, COX-2, TGF-β1, and α-SMA. The expression levels of caspase-3 and Bax were increased significantly, whereas Bcl-2 expression was decreased. In conclusion, the phosphorylation of PI3K/Akt/mTOR was increased in the kidney of DOCA-salt hypertensive rats.
Deoxycorticosterone acetate (DOCA)-salt hypertension in animals is an established model of volume-dependent hypertension with renal injury. It is induced in animals by the implantation of DOCA strips, which introduce high levels of mineralocorticoid that mimic aldosterone overload.1 Although mineralocorticoid is classically known to regulate renal tubular sodium reabsorption in the collecting duct, recent data indicate that it also causes inflammation and fibrosis in the kidney and cardiovascular system.2,3 It is well known that DOCA-salt hypertension is accompanied by renal injury, such as mesangial expansion, glomerulosclerosis, and tubulointerstitial fibrosis.4
The mammalian target of rapamycin (mTOR) is a ubiquitously expressed intracellular serine/threonine protein kinase that plays a crucial role in regulating cell proliferation and organ growth through effects on many cellular processes.5 Recently, it was suggested that activation of the mTOR pathway is involved in the glomerular hypertrophy associated with the loss of function in nephrons, interstitial fibrosis, and tubular atrophy that occur in several forms of kidney disease.6 In addition, the mineralocorticoid receptor-mediated activation of the mTOR signaling pathway results in cellular proliferation in human mesangial cells.7 However, the role of the mTOR signaling pathway in the pathogenesis of kidney injury in mineralocorticoid hypertension remains unclear. We therefore investigated the role of the mTOR signaling pathway in the pathogenesis of renal injury in DOCA-salt hypertension in this study.
The use of animals in this study was approved by the Ethics Committee of Chonnam National University Medical School, and the experimental procedure conformed to the Institutional Guidelines for Experimental Animal Care and Use. Male Sprague-Dawley rats weighing 200 to 250 g were implanted with DOCA strips (200 mg/kg) 1 week following unilateral nephrectomy. Control rats were unilaterally nephrectomized without DOCA. Rats were then supplied with 0.9% saline to drink. Four weeks after DOCA implantation, systolic blood pressure (SBP) was measured by the tail-cuff method, and the rats were euthanized. Kidneys were then excised and processed for semiquantitative immunoblotting.
Whole kidneys were homogenized in ice-cold isolation solution, pH 7.2 (0.3 M sucrose, 25 mM imidazole, 1 mM EDTA, 8.5 µM leupeptin, 1 mM phenylmethylsulfonyl fluoride), and centrifuged at 1,000×g for 15 min at 4℃ to remove whole cells, nuclei, and mitochondria. The total protein concentration was measured by use of the Pierce BCA Protein Assay Reagent Kit (Pierce, Rockford, IL), and all samples were adjusted to the same final protein concentration with isolation solution. Samples were then solubilized in SDS-containing sample buffer for 15 min at 65℃ before they were stored at -20℃. SDS-PAGE was performed on 9% or 12% polyacrylamide gels by using the Bio-Rad Mini Protean II system (Bio-Rad, Hercules, CA, USA) and the proteins were transferred onto nitrocellulose membranes (Hybond ECL RPN3032D, Amersham Pharmacia Biotech, Little Chalfont, UK). Membranes were subsequently blocked with 5% milk in phosphate-buffered saline with Tween 20 (PBST), pH 7.5 (80 mM Na2HPO4, 20 mM NaH2PO4, 100 mM NaCl, 0.1% Tween 20), for 1 h. After an overnight incubation with 1:1000 dilutions of primary antibodies at 4℃, membranes were incubated with 1:1,500 dilutions of secondary anti-rabbit (P448, DAKO, Glostrup, Denmark) or anti-mouse (P447, DAKO, Glostrup, Denmark) horseradish peroxidase-conjugated antibodies. Proteins were visualized by using an enhanced chemiluminescence system.
Affinity-purified anti-rabbit antibodies against phosphorylated phosphatidylinositol-3-kinase (p-PI3K), phosphorylated Akt (p-Akt), phosphorylated mTOR (p-mTOR), caspase-3, Bax, and Bcl-2 were obtained from Cell Signaling Technology (Beverly, MA, USA). Other antibodies were obtained from the following suppliers: cyclooxygenase-2 (COX-2) antibodies were obtained from Cayman Chemical (Ann Arbor, MI, USA), transforming growth factor-β1 (TGF-β1) antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA, USA), anti-mouse antibodies against ED-1 were from Serotec (Raleigh, NC, USA), and α-smooth muscle actin (SMA) was from Sigma Chemical Co. (St. Louis, MO, USA).
Four weeks after DOCA implantation, SBP was significantly increased in DOCA-implanted rats compared with control rats (233±15 mmHg vs. 127±6 mmHg, respectively, p<0.05). The ratio of kidney weight to body weight was also increased (8.49±1.67 mg/g vs. 4.91±0.48 mg/g, p<0.05).
Fig. 1 and Table 1 depict changes in the phosphorylation state of mTOR signaling pathway proteins elicited by DOCA-salt hypertension. The expression levels of p-PI3K, p-Akt, and p-mTOR were significantly increased in the kidney in DOCA-salt hypertensive rats compared with the control rats. When the expression levels of inflammatory cytokines were examined, the expression of both ED-1 and COX-2 had increased in the kidney after treatment with DOCA-salt (Table 1). Likewise, the expression of TGF-β1, a pro-fibrotic molecule derived from inflammatory cells, and α-SMA, a molecular marker for myofibroblasts, was significantly increased in the kidney of DOCA-salt hypertensive rats (Fig. 2 and Table 1). Finally, the effects of DOCA-salt hypertension on apoptosis were examined by determining the expression levels of the pro-apoptotic molecular markers caspase-3 and Bax and the anti-apoptotic Bcl-2 protein. It was found that the expression of both pro-apoptotic proteins was significantly increased in the kidneys of DOCA-salt hypertensive rats compared with control rats, whereas the renal expression of Bcl-2 was decreased in rats after treatment with DOCA-salt (Fig. 3 and Table 1).
We have previously demonstrated that DOCA-salt-treated rats exhibit marked hypertension with impaired renal function, including decreased creatinine clearance, polyuria with decreased urine osmolality, renal hypertrophy, and proteinuria.8-10 In the present study, we showed that severe DOCA-salt hypertension also results in increased kidney weight.
It was previously shown that DOCA-salt treatment results in glomerulosclerosis and tubulointerstitial fibrosis, which is related to increased infiltration of inflammatory cells, increased expression of pro-inflammatory and pro-fibrotic molecules, and the epithelial-mesenchymal transition (EMT).9-11 When the expression of the inflammatory markers ED-1 and COX-2 was examined in the kidney in our rat model of DOCA-salt hypertension, it was found to be increased compared with that of control rats, as was the expression of TGF-β1 and α-SMA. TGF-β itself plays a crucial role in the pathogenesis of progressive renal disease, in which renal fibrosis is promoted by the accumulation of extracellular matrix. TGF-β may also induce the production of pro-apoptotic molecules such as caspase-3 and Bax, as well induce EMT.12,13 Our data showed that the renal expression of the pro-apoptotic proteins caspase-3 and Bax was increased and that of the anti-apoptotic factor Bcl-2 was decreased in the DOCA-salt model of hypertension. These changes may contribute to the renal injury that occurs in DOCA-salt hypertension; however, the upstream mechanism of these changes remains elusive.
It was previously demonstrated that activation of the mineralocorticoid receptor can stimulate the PI3K/Akt/mTOR pathway in human mesangial cells.7 Therefore, in this study, we used an established rat model in which DOCA-salt induces mineralocorticoid hypertension to investigate the changes that occur in the PI3K/Akt/mTOR signaling pathway in the kidney as a result of this state. This pathway leads to the phosphorylation and activation of mTOR, which plays a role in mediating cell size and mass, proliferation, and survival.14,15 In our study, PI3K, Akt, and mTOR proteins were all found to have increased phosphorylation levels in the kidneys of DOCA-salt hypertensive rats. Recent data have further revealed that the activation of the PI3K/Akt/mTOR pathway in the kidney results in glomerular hypertrophy, tubulointerstitial atrophy, and fibrosis in several animal models of kidney disease.5,6 Because renal hypertrophy may contribute to podocyte injury, proteinuria, and progressive loss of renal function, it is an important structural change that occurs in progressive renal disease.16 The activation of mTOR plays a pivotal role in the pathogenesis of renal hypertrophy in cases of compensatory renal hypertrophy and diabetic nephropathy.17-19 The activation of mTOR may also promote the tubular injury, atrophy, and interstitial fibrosis that occurs in response to interstitial inflammation and EMT following the stimulation of lymphocyte and fibroblast proliferation.20-22 In conclusion, our finding that the phosphorylation of PI3K/Akt/mTOR was increased in the kidneys of DOCA-salt hypertensive rats suggests that the mTOR signaling pathway is related to the pathogenesis of DOCA-salt hypertension with renal injury.
Figures and Tables
FIG. 1
Expression of phosphorylated phosphatidylinositol-3-kinase (p-PI3K), phosphorylated Akt (p-Akt), and phosphorylated mammalian target of rapamycin (p-mTOR) in the kidney. DOCA, deoxycorticosterone acetate. *p<0.05 compared with the control.
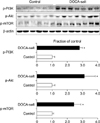
FIG. 2
Expression of transforming growth factor (TGF)-β1 and α-smooth muscle actin (SMA) in the kidney. DOCA: deoxycorticosterone acetate. *p<0.05 compared with the control.
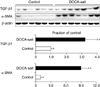
FIG. 3
Expression of caspase-3, Bax, and Bcl-2 in the kidney. DOCA: deoxycorticosterone acetate. *p<0.05 compared with the control.
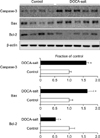
TABLE 1
Summary of immunoblotting results in the kidneys from control and DOCA-salt hypertensive rats

Values are expressed as mean±SEM. DOCA: deoxycorticosterone acetate, p-PI3K: phosphorylated phosphatidylinositol-3-kinase, p-Akt: phosphorylated Akt, p-mTOR: phosphorylated mammalian target of rapamycin, TGF-β1: transforming growth factor-β1, α-SMA: α-smooth muscle actin, COX-2: cyclooxygenase-2. *p<0.05 compared with the control.
References
1. Gavras H, Brunner HR, Laragh JH, Vaughan ED Jr, Koss M, Cote LJ, et al. Malignant hypertension resulting from deoxycorticosterone acetate and salt excess: role of renin and sodium in vascular changes. Circ Res. 1975. 36:300–309.

2. Iglarz M, Touyz RM, Viel EC, Amiri F, Schiffrin EL. Involvement of oxidative stress in the profibrotic action of aldosterone. Interaction wtih the renin-angiotension system. Am J Hypertens. 2004. 17:597–603.

3. Sun Y, Zhang J, Lu L, Chen SS, Quinn MT, Weber KT. Aldosterone-induced inflammation in the rat heart : role of oxidative stress. Am J Pathol. 2002. 161:1773–1781.
4. Lijnen PJ, Petrov VV, Fagard RH. Association between transforming growth factor-beta and hypertension. Am J Hypertens. 2003. 16:604–611.
5. Liu Y. Rapamycin and chronic kidney disease: beyond the inhibition of inflammation. Kidney Int. 2006. 69:1925–1927.

6. Lieberthal W, Levine JS. The role of the mammalian target of rapamycin (mTOR) in renal disease. J Am Soc Nephrol. 2009. 20:2493–2502.

7. Huang S, Zhang A, Ding G, Chen R. Aldosterone-induced mesangial cell proliferation is mediated by EGF receptor transactivation. Am J Physiol Renal Physiol. 2009. 296:F1323–F1333.

8. Bae EH, Kim IJ, Ma SK, Kim SW. Altered regulation of renal sodium transporters and natriuretic peptide system in DOCA-salt hypertensive rats. Regul Pept. 2009. 157:76–83.

9. Bae EH, Kim IJ, Park JW, Ma SK, Lee JU, Kim SW. Renoprotective effect of rosuvastatin in DOCA-salt hypertensive rats. Nephrol Dial Transplant. 2010. 25:1051–1059.

10. Bae EH, Kim IJ, Ma SK, Kim SW. Rosiglitazone prevents the progression of renal injury in DOCA-salt hypertensive rats. Hypertens Res. 2010. 33:255–262.

11. Iwazu Y, Muto S, Hirahara I, Fujisawa G, Takeda S, Kusano E. Matrix metalloproteinase 2 induces epithelial-mesenchymal transition in proximal tubules from the luminal side and progresses fibrosis in mineralocorticoid/salt-induced hypertensive rats. J Hypertens. 2011. 29:2440–2453.

12. Miyajima A, Chen J, Lawrence C, Ledbetter S, Soslow RA, Stern J, et al. Antibody to transforming growth factor-beta ameliorates tubular apoptosis in unilateral ureteral obstruction. Kidney Int. 2000. 58:2301–2313.

13. Park JW, Bae EH, Kim IJ, Ma SK, Choi C, Lee J, et al. Paricalcitol attenuates cyclosporine-induced kidney injury in rats. Kidney Int. 2010. 77:1076–1085.

14. Katso R, Okkenhaug K, Ahmadi K, White S, Timms J, Waterfield MD. Cellular function of phosphoinositide 3-kinases: implications for development, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2001. 17:615–675.

17. Chen JK, Chen J, Neilson EG, Harris RC. Role of mammalian target of rapamycin signaling in compensatory renal hypertrophy. J Am Soc Nephrol. 2005. 16:1384–1391.

18. Mariappan MM, Feliers D, Mummidi S, Choudhury GG, Kasinath BS. High glucose, high insulin, and their combination rapidly induce laminin-beta1 synthesis by regulation of mRNA translation in renal epithelial cells. Diabetes. 2007. 56:476–485.

19. Sataranatarajan K, Mariappan MM, Lee MJ, Feliers D, Choudhury GG, Barnes JL, et al. Regulation of elongation phase of mRNA translation in diabetic nephropathy: amelioration by rapamycin. Am J Pathol. 2007. 171:1733–1742.

20. Lloberas N, Cruzado JM, Franquesa M, Herrero-Fresneda I, Torras J, Alperovich G, et al. Mammalian target of rapamycin pathway blockade slows progression of diabetic kidney disease in rats. J Am Soc Nephrol. 2006. 17:1395–1404.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download