INTRODUCTION
Drug-eluting stents (DES) have greatly reduced in-stent restenosis by inhibiting neointimal growth. Recently, however, stent fracture has emerged as a complication associated with in-stent restenosis, stent thrombosis, and aneurysm formation.1,2 Most stent fractures are associated with sirolimus-eluting stent (SES) implantation in the right coronary artery. Stent fractures are extremely rare with a paclitaxel-eluting stent or zotarolimus-eluting stent (ZES) in the left anterior descending artery (LAD) or in a left circumflex artery lesion.3
Here we describe a case of a stent fracture that occurred with a ZES that had been implanted in a calcified LAD lesion in a patient undergoing hemodialysis due to chronic renal insufficiency.
CASE REPORT
A 44-year-old male patient was admitted with a history of effort-related chest pain for several months. He had a history of diabetes mellitus for 8 years and chronic renal insufficiency with hemodialysis for 3 years. The ECG showed left ventricular hypertrophy without ST segment change.
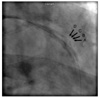
Coronary angiography revealed severe calcified eccentric stenosis in the LAD (Fig. 1). The LAD was engaged with a 6-Fr guiding catheter (JL4, Cordis, Miami Lakes, FL, USA), predilatation was performed with a Sprinter 2.5×20 mm balloon (Medtronic, Minneapolis, MN, USA), and a ZES (Endeavor 3.0×30 mm, Medtronic) was deployed at 12 atm. Additional high-pressure ballooning with a 3.0×12 mm balloon (Quantum, Boston Scientific, Natick, MA, USA) at 16 atm showed good angiographic results (Fig. 2). After the procedure, the patient remained asymptomatic, and he was discharged and followed up through the outpatient department.
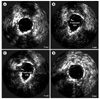
Eight months after the procedure, new recurrent chest pain developed during hemodialysis despite full anti-anginal medication, and follow-up coronary angiography was performed. Mild in-stent restenosis was found in the middle of the previously implanted ZES, and fluoroscopic images showed that the ZES had a gap in the middle of the stent indicating a stent fracture (Fig. 3). Intravascular ultrasonography study (IVUS) with an automated pullback speed of 0.5 mm/s was performed, and the IVUS revealed partial absence of the stent struts in the middle of the stent, corresponding to the area of the stent fracture (Fig. 4). The external elastic lamina area was 4.82 mm2, the stent area was 4.56 mm2, the minimum lumen area was 4.21 mm2, the neointimal area was 0.47 mm2, and the percentage of neointimal area was 28%.
Although the minimal luminal area was over 4 mm2 and the percentage of neointimal area was small, we decided on a stent-in stent strategy because of the patient's recurrent chest pain after full anti-anginal medication and chose another kind of open cell designed stent. A paclitaxel-eluting stent (Taxus Liberte 3.0×12 mm, Boston Scientific) was deployed at the fractured site at nominal pressure. Final coronary angiography showed good results, and IVUS showed no discontinuation of the stent struts.
The patient was discharged with antiplatelet medication. No adverse cardiac events were noted during the 24 months of clinical follow-up, and the patient remains asymptomatic on medications.
DISCUSSION
Stent fracture is rare but is considered to be an important cause for stent thrombosis, re-intervention, and myocardial infarction after DES implantation.4,5 Increased mechanical stresses on the stent predispose towards stent fracture. Factors such as a long, extremely angular and calcified lesion; post-dilatation with high pressure; and increased wall motion along the right coronary artery are likely to be contributing risk factors of stent fracture.6,7
Sirolimus-eluting stent implantation is another independent predictor of stent fracture, and most cases have been reported with SES.8,9 The closed cell design of the SES is less deformable in response to dynamic loading imposed by the movement of coronary arteries and makes a weak point at which a stent fracture may develop. However, stent fracture has also been reported with paclitaxel-eluting stents or ZES, although it is extremely rare,10 and there have been no reported cases in the LAD in a hemodialyzed patient.
Revascularization of a stent fracture is controversial because there is a possibility of recurrence of the stent fracture and the outcome of the stent fracture lesion is thought to be poor. In this case, we performed revascularization because the patient complained of angina and there was a risk of stent thrombosis or localized aneurysm formation.11-14 We successfully treated the patient with a different kind of open-cell-designed short stent implantation. We chose the SES because the closed-cell design tends to fracture by the movement of coronary arteries. Also, because we thought that the oversized, high-pressure ballooning may have contributed to stent fracture, we did not use high-pressure ballooning during the second percutaneous coronary intervention, and the patient has remained asymptomatic during 9 months of clinical follow-up.
Although stent fractures appear to be more frequent after SES implantation and in the right coronary artery, they can occur in any kind of DES and in any part of the coronary arteries, as in the present patient. Because clinical data on stent fractures are limited, future large multicenter studies will be required to determine the exact mechanisms and long-term management of stent fracture.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download