Abstract
Sphingosine-1-phosphate (S1P) is emerging as a new class of second messenger involved in cellular proliferation, differentiation, and apoptosis and is implicated in diverse physiological functions. Despite many studies on the biological functions of S1P, however, little is known about its role in neuronal differentiation. By use of reverse transcription-polymerase chain reaction and immunostaining, this study aimed to explore whether S1P can differentiate neuroblastoma cells into neural cells. After incubation with 1 uM or 10 uM S1P, the number of neurite-bearing cells increased. Furthermore, the neuroblastoma cells revealed immunoreactivity for neural-specific markers such as GAP43, NFH, and SYP by immunostaining. The expression of NFH, MAP2, SYP, NeuroD1, and SYT mRNA, which is specific for neurons, was increased as shown by RT-PCR studies. The results of this study suggest that that S1P can induce neuronal differentiation and may be a good candidate for the treatment of neurodegenerative diseases.
Sphingosine-1-phosphate (S1P) is derived from sphingosine, the backbone of most sphingolipids, and is now emerging as a vital lipid mediator.1 S1P is one of a new class of second messengers involved in cellular proliferation, differentiation, and apoptosis and implicated in diverse physiological functions, including immune modulation, vascular and nervous system development, regulation of smooth muscle, and auditory and vestibular function.1-4
Intermediates in the biosynthesis and catabolism of sphingolipids, ceramide, sphingosine, and S1P have recently been implicated in the intracellular signaling important for neuronal survival, differentiation, development, and death. Because increased levels of ceramide were observed during differentiation of neuroblastoma Neuro2a cells and SH-SY5Y cells, it has been suggested that ceramide plays a role in neuronal differentiation.2,5,6 Also, S1P was recently shown to be a key regulator of proliferation and differentiation by up-regulating sphingosine kinase expression in retina photoreceptors.7
Neuroblastomas derived from immature sympathetic ganglionic cells are arrested at various stages of differentiation.8 Differentiation studies have revealed that neuroblastoma cell lines can be induced to differentiate in the presence of various agents and growth factors. At first, human SH-SY5Y neuroblastoma cells (a subclone of the SKN-SH cell line) were reported to differentiate morphologically and biochemically in response to bioactive phorbolesters.9
Despite many studies concerning the physiologic actions of S1P, little is known about its capacity to differentiate neurons. This study intended to explore the differentiating action of S1P by using neuroblastoma cell lines. In this study, S1P induced neuronal differentiation in neuroblasoma cells.
SH-SY5Y human neuroblastoma cells (ATCC number: CRL-2266™) capable of differentiation to neuronal cells under specific conditions were cultured in DMEM (Hyclone, Logan, UT, USA) containing 10% fetal bovine serum (Hyclone) and 1% penicillin-streptomycin (Hyclone). The cells were grown to approximately 80% confluence in a 37℃ humidified incubator in an environment of 5% CO2 and 95% air and were then harvested in trypsin containing EDTA (Hyclone). Sphingosine-1-phosphate (Sigma Chemical Co., St. Louis, MO, USA) was initially dissolved and diluted to a final stock concentration of 10 mM in water before use. The SH-SY5Y neuroblastoma cells were differentiated for 3 or 4 days in the presence of 1 or 10 uM S1P.
The cells were grown under conditions with S1P for 4 days. The morphology of the SH-SY5Y cells was investigated with a NiKon phase-contrast inverted microscope equipped with a Nikon Coolpix 4500 high-resolution camera. Changes in neurite length were observed over 4 days, and the medium was replaced every 2 days. A total of 200 to 300 cells were microscopically evaluated and scored for neurite formation by use of the Image J program if they had a neurite that was longer than one cell diameter or had a growth cone. All experiments were repeated at least three times with similar results.
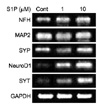
Cells were cultured in 6-well plates as described previously. Total RNA was extracted from the cultured cells by using the Tri Reagent (Molecular Research Center Inc., Cincinnati, OH, USA) isolation reagent. cDNA was synthesized by reverse transcription with M-MLV reverse transcriptase (Gibco BRL, Grand Island, NY, USA) and 1 mmol total RNA. The cDNA was amplified by 25 to 35 cycles of PCR (Takara Bio Inc., Shiga, Japan) with Ex-Taq polymerase (Takara Bio Inc.). The primers (Bioneer Co., Daejeon, Korea) used were chosen and checked for specificity by using a basic BLAST search. The RT-PCR products were separated electrophoretically on 2% agarose gels. We used the following primer pairs (gene, forward, reverse, and cDNA product length): 5'-AAA TCG CAT CCA GAT TTT C-3', 5'-CAC TGC CTC CTA GCT TGT C-3', 316 bp; neurofilament-H (NFH), 5'-CCG ACG ACA CCA AGC TCA-3', 5'-GGA ATG AAA CAG GGC GTT-3', 336 bp; microtubule-associated protein-2 (MAP-2), 5'-TGC CAT CTT GGT GCC GA-3', 5'-CTT GAC ATT ACC ACC TCC AGG T-3', 460 bp; synaptophysin (SYP), 5'-CTT CCT GCA GAA CAA GTA CC-3', 5'-CTT AAA CAC GAA CCA CAG GT-3', 295 bp; neurogenic differentiation 1 (NeuroD1), 5'-CCG ACA GAG CCC AGA TGT AGT TCT T-3', 5'-GCC CCA GGG TTA TGA GAC TAT CAC T-3', 278 bp; synaptotagamin (SYT), 5'-CGC AAA CTG GGC AAA CGC TA-3', 5'-GCA ACC CTC GTG GGC CTC-3', 421 bp; GAPDH, 5'-CAT GAC CAC AGT CCA TGC CAT CAC T-3', 5'-TGA GGT CCA CCA CCC TGT TGC TGT A-3', and 461 bp.
Immunochemical determination of neuronal differentiated SH-SY5Y cells was performed as follows. The cells were grown on poly-D-lysine-coated aclar plastic coverslips for 4 days in the presence of 10 mM S1P, fixed for 15 min with 4% paraformaldehyde (Sigma Chemical Co.), permeabilized for 20 min with 0.3% Triton X-100 (Sigma Chemical Co.) including 10% normal goat serum (Vector Laboratories Inc., Burlingame, CA, USA) in phosphate-buffered saline, and incubated with antibodies specific for each antigen marker. The cultures incubated with primary antibodies were followed by biotinylated secondary antibodies and avidin-biotin complex (Vector Laboratories Inc.). The cell type-specific markers used were growth associated protein-43 (GAP43), NFH, and SYP. The antibodies were purchased from Chemicon (Chemicon, Temecula, CA, USA).
After incubation with 1 uM or 10 uM S1P, the neuroblastoma cells revealed neurite outgrowth. The number of neurite-bearing cells increased from 14% to 37% and 28% by 1 uM S1P after 72 hours and 96 hours, respectively. The number of neurite-bearing cells increased from 14% to 18% and 46% by 10 uM S1P after 72 hours and 96 hours, respectively (Fig. 1).
After incubation with S1P, the neuroblasoma cells revealed immunoreactivity for neural-specific markers. The neuronal cells were positive for neuronal markers such as GAP43, NFH, and SYP (Fig. 2).
Reverse transcription-polymerase chain reaction was used to examine the expression of mRNA related to neuronal differentiation. The expression of NFH, Ngn1, MAP2, SYP, NeuroD1, and SYT mRNA, which is specific for neurons, was increased by treatment with S1P (Fig. 3).
Neuronal differentiation and cellular homeostasis are fundamental events in the development of the nervous system as well as in the regeneration of damaged nervous tissue. Differentiation is regulated by a complex mechanism, in which the result of several interactions between the cell and the extracellular medium determines the fate of the cellular function.10
S1P is known to induce differentiations in various cell types. It stimulates differentiation of adipose tissue-derived mesenchymal stem cells towards smooth muscle cells. It increases the expression of smooth muscle cell-specific proteins such as alpha-smooth muscle actin (alpha SMA) and transgelin.11 Sphingosine kinase, which promotes the formation of S1P, enhances the expression of myogenic differentiation markers in myoblasts.12 Recently, Miranda et al proposed S1P as a key regulator in the development of photoreceptors. It increases the proliferation of photoreceptor progenitors and increases the formation of apical processes and enhances the expression of opsin and peripherin in photoreceptors.13
Despite their tumoral origin, neuroblastoma cell lines can be induced to differentiate in vitro by several agents.14 In this work, we used the SH-SY5Y neuroblastoma cell line, which showed homogeneous populations of differentiated cells due to STP or retinoic acid.15 Retinoic acids are natural and synthetic derivatives of vitamin A that, together with their nuclear control genetic programs, are essential for embryonic development, organ homeostasis, cell growth, differentiation, and apoptosis.16
We observed that treatment with S1P for 4 days induced neuronal morphological changes in neuroblastoma cells, which were similar to those described by other authors.15,16 To confirm that the morphological changes observed actually resulted from the neuronal differentiation, the expression of several neuronal markers was explored. We observed that the typical morphological feature of differentiation was accompanied by biochemical changes. In the present study, S1P induced immunoreactivities for neuronal markers and increased the expression of neuronal gene markers in neuroblastoma cells.
Intermediates in the biosynthesis and catabolism of sphingolipids, ceramide, sphingosine, and S1P have recently been implicated in intracellular signaling important for neuronal survival, differentiation, development, and death. S1P is emerging as a new class of second messenger involved in cellular proliferation, differentiation, and apoptosis.2 The above experimental results imply that S1P could be used to differentiate neuronal cells and may be a good candidate for the treatment of neurodegenerative diseases.
Figures and Tables
FIG. 1
Effects of S1P on the number of neurite-bearing cells. After incubation with S1P, neuroblastoma cells revealed morphological changes with neurite outgrowth (top), and the number of neurite-bearing cells was increased dose-dependently (bottom). Magnification is 10×10. Statistical significance is *p<0.05.
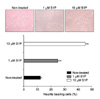
FIG. 2
Immunocytochemical staining for neuron-specific markers in differentiated cells by S1P. Neuroblastoma cells were induced to differentiate into neuronal cells in the presence of S1P. Immunocytochemistry revealed that the expression of neuron-specific markers such as GAP43, NFH, and SYP was increased. Scale bar represents 50 µm (10×10).

ACKNOWLEDGEMENTS
This study was supported by a grant from the Chonnam National University Hospital Research Institute of Clinical Medicine (CRI 08053-1).
References
1. Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003. 4:397–407.

2. Edsall LC, Pirianov GG, Spiegel S. Involvement of sphingosine 1-phosphate in nerve growth factor-mediated neuronal survival and differentiation. J Neurosci. 1997. 17:6952–6960.

3. Brinkmann V. Sphingosine 1-phosphate receptors in health and disease: mechanistic insights from gene deletion studies and reverse pharmacology. Pharmacol Ther. 2007. 115:84–105.

4. Choi JW, Lee CW, Chun J. Biological roles of lysophospholipid receptors revealed by genetic null mice: an update. Biochim Biophys Acta. 2008. 1781:531–539.

5. Riboni L, Prinetti A, Bassi R, Caminiti A, Tettamanti G. A mediator role of ceramide in the regulation of neuroblastoma Neuro2a cell differentiation. J Biol Chem. 1995. 270:26868–26875.

6. Rius RA, Edsall LC, Spiegel S. Activation of sphingosine kinase in pheochromocytoma PC12 neuronal cells in response to trophic factors. FEBS Lett. 1997. 417:173–176.

7. Miranda GE, Abrahan CE, Politi LE, Rotstein NP. Sphingosine-1-phosphate is a key regulator of proliferation and differentiation in retina photoreceptors. Invest Ophthalmol Vis Sci. 2009. 50:4416–4428.

9. Påhlman S, Odelstad L, Larsson E, Grotte G, Nilsson K. Phenotypic changes of human neuroblastoma cells in culture induced by 12-O-tetradecanoyl-phorbol-13-acetate. Int J Cancer. 1981. 28:583–589.

10. Labbaye C, Valtieri M, Barberi T, Meccia E, Masella B, Pelosi E, et al. Differential expression and functional role of GATA-2, NF-E2, and GATA-1 in normal adult hematopoiesis. J Clin Invest. 1995. 95:2346–2358.

11. Medlin MD, Staus DP, Dubash AD, Taylor JM, Mack CP. Sphingosine 1-phosphate receptor 2 signals through leukemia-associated RhoGEF (LARG), to promote smooth muscle cell differentiation. Arterioscler Thromb Vasc Biol. 2010. 30:1779–1786.

12. Meacci E, Nuti F, Donati C, Cencetti F, Farnararo M, Bruni P. Sphingosine kinase activity is required for myogenic differentiation of C2C12 myoblasts. J Cell Physiol. 2008. 214:210–220.

13. Rotstein NP, Miranda GE, Abrahan CE, German OL. Regulating survival and development in the retina: key roles for simple sphingolipids. J Lipid Res. 2010. 51:1247–1262.

14. Yuste VJ, Sánchez-López I, Solé C, Encinas M, Bayascas JR, Boix J, et al. The prevention of the staurosporine-induced apoptosis by Bcl-X(L), but not by Bcl-2 or caspase inhibitors, allows the extensive differentiation of human neuroblastoma cells. J Neurochem. 2002. 80:126–139.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download