Abstract
Hemophagocytic lymphohistiocytosis (HLH) is an unusual syndrome characterized by fever, hepatosplenomegaly, cytopenias, hypertriglyceridemia, hypofibrinogenemia, and pathologic findings of hemophagocytosis in the bone marrow and other tissues. HLH may be familial or associated with different types of infections, autoimmune disorders, or malignancies. Infection-associated HLH has been reported in various viral, bacterial, fungal, and parasitic infections, and case reports of parasitic infections implicated in HLH include rare cases from Plasmodium vivax infection, which occasionally affects both military personnel and civilians in Korea. We describe an unusual case of HLH resulting from Plasmodium vivax infection and review the literature. This case suggests that clinical suspicion of HLH is important when P. vivax infection is accompanied by cytopenias. Administration of antimalarial drugs may prevent irreversible end organ damage resulting from P. vivax-associated HLH.
Hemophagocytic lymphohistiocytosis (HLH) is a rare syndrome characterized by reactive, systemic proliferation of benign histiocytes throughout the reticuloendothelial system.1 HLH results from impaired functions of natural killer (NK) and cytotoxic T cells, whereas activities of lymphocytes and histiocytes are augmented, leading to phagocytosis of hematopoietic cells.1 HLH comprises two different conditions that may be difficult to distinguish from one another. The primary autosomal recessive form, familial hemophagocytic lymphohistiocytosis, is a fatal disease with its onset during infancy or early childhood and has a median survival of less than 2 months if untreated.2 Secondary HLH may develop as a result of strong immunological activation of the immune system, which may be caused by a severe infection. Among the infections, viral pathogens reported to be associated with HLH include Epstein-Barr virus, cytomegalovirus, parvovirus, herpes simplex virus, varicella-zoster virus, measles virus, human herpes virus-8, and human immunodeficiency virus, alone or in combination.1 HLH may also coincide with various bacterial infections, parasite infections, and fungal infections.1
It is important to realize that early therapeutic interventions are required when strong clinical suspicion of infection-associated HLH is present to prevent irreversible end organ damage. Our report describes an unusual case of HLH associated with Plasmodium vivax infection, which was treated successfully with antimalarial agents. We also review the literature on P. vivax-associated HLH.
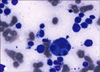
A 64-year-old woman living in Gimpo, Gyeonggi Province, visited the emergency room of Seoul St. Mary's Hospital, South Korea. She complained of chills followed by fever up to 40℃ for several hours occurring every two days and diffuse abdominal pain for 7 days. She had not taken any medication except that to treat reflux esophagitis. She had no history of traveling or contact with foreigners. Her vital signs were as follows: blood pressure 130/70 mmHg, heart rate 96/min, temperature 38.2℃, and respiratory rate 24/min. She appeared acutely ill and hepatosplenomegaly was noted, but there were no palpable lymph nodes. Her leukocyte count was 2,380/mm3 (4,000-10,000/mm3), her absolute neutrophil count was 830/mm3, her hemoglobin was 11.7 g/dl (13.0-18.0 g/dl), her platelet count was 36,000/mm3 (150,000-450,000/mm3), and her reticulocyte count was 0.52% (0.2-2%). Blood chemistry revealed aspartate aminotransferase of 39 U/L (14-40 U/L), alanine aminotransferase of 30 U/L (9-45 U/L), total bilirubin of 3.97 mg/dl (0.47-1.58 mg/dl), blood urea nitrogen of 15.3 mg/dl (7.0-20.0 mg/dl), creatinine of 0.94 mg/dl (0.6-1.2 mg/dl), lactate dehydrogenase of 1,451 U/L (250-450 U/L), triglyceride of 210 mg/dl (40-200 mg/dl), and ferritin of 923 µg/L (20-300 µg/L). The fibrinogen assay showed >500 mg/dl (160-350 mg/dl). Cultures of blood and urine were done twice at 1-hour intervals, and all of the results were negative. Antibodies against viral pathogens including Epstein-Barr virus (EBV), cytomegalovirus (CMV), and herpes simplex virus (HSV) were not detected in the serum. Specifically, HSV IgM, HSV IgG, CMV IgM, EBV early antigen IgG, and EBV viral capsid antigen (VCA) IgM were negative, but CMV IgG, EBV VCA IgG, and EBV nuclear antigen IgG were positive. Also, serologic tests for hepatitis A, B, and C viruses and human immunodeficiency virus were negative. Additional serologic tests for Leptospira, Orientia tsutsugamushi, Haantan virus, and connective tissue diseases were also negative, but trophozoites and gametocytes of P. vivax were observed in a peripheral blood smear (Fig. 1), and the parasitemia value was 1,540/µL. Subsequent positive malaria polymerase chain reaction confirmed the diagnosis of P. vivax infection. A computed tomographic scan of the abdomen showed moderate hepatosplenomegaly. Bone marrow aspirate revealed rare intracellular parasites with hypocellular bone marrow, but proliferation of histiocytes with prominent hemophagocytosis was noted (Fig. 2). Subsequent immunohistochemical stain for Epstein-Barr virus was negative. Diagnosis of P. vivax malaria-associated HLH was made, and hydroxychloroquine (25 mg/kg) was administered for 3 days, followed by primaquine (15 mg per day) for 14 days. The patient recovered completely with the antimalarial medication, and all the abnormal laboratory findings and follow-up peripheral blood smear and bone marrow aspirate were normalized on the 12th day of hospitalization without additional implementation of immunosuppressive agents.
The Histiocyte Society HLH study group revised the diagnostic guidelines of HLH in 2004.3 According to them, the diagnosis of HLH is established either by a molecular diagnosis consistent with HLH (primary HLH) or by having five out of eight of the following: 1) fever; 2) splenomegaly; 3) cytopenia affecting more than two cell lineages; 4) hypertriglyceridemia and/or hypofibrinogenemia; 5) hemophagocytosis in the bone marrow, spleen, or lymph nodes without evidence of malignancy; 6) low or absent natural killer cell cytotoxicity; 7) hyperferritinemia; and 8) elevated soluble CD25.3 In our report, the patient presented with fever, hepatosplenomegaly, cytopenia of more than 2 cell lineages, hyperferritinemia, and hemophagocytosis in the bone marrow aspirates, meeting the diagnostic criteria for HLH (Table 1).
Secondary HLH may occur most commonly in the setting of malignancy, autoimmune disease, drug hypersensitivity reaction, or infection.1 Infection-associated HLH has been reported in a great variety of viral, bacterial, fungal, and, rarely, parasitic infections.1 P. vivax, a causative agent of relapsing tertian human malaria, is the second most important human malaria and affects several hundred million people annually. It has the widest geographic distribution throughout the world, and is usually found in Central and South America, India, and Southeast Asia.4 Presently, P. vivax is the only endemic malarial species in Korea.
A search of the English and Korean language literature revealed seven other cases of P. vivax-associated HLH.4-8 Of these cases, patients' data were available for four cases, including our case (Table 2).4-6 In all four cases, including our case, treatment of P. vivax malaria was sufficient for the patients to recover, and no immunosuppressive agents were applied in any of these cases.4-6
The possible immunological mechanism of HLH may be excessive production of Th1 cytokines, such as interferon-gamma, tumor necrosis factor-alpha, interleukin-1, or interleukin-6, from activated mononuclear cells.9 Patients with Epstein-Barr virus-associated HLH, which can be fatal due to hemorrhage, infection, or multi-organ failure, may be required to be treated with a combination of immunosuppressive agents.10 Otherwise, patients with infection-associated HLH with other pathogens can usually recover with supportive care and treatment targeted to the underlying infection.10 Unless associated with underlying immunodeficiency, the outcome for HLH caused by parasitic infections, including P. vivax infection, is generally good when antimalarial drugs are properly applied, as occurred in our patient.4-6
When a patient with bicytopenia or pancytopenia presents with fever or documented infection, the possibility of infection-associated HLH should be raised. In particular, systemic parasitic infections, such as malaria, should be included in the differential diagnosis of pancytopenia and infection-associated HLH. Laboratory tests to confirm the etiologic pathogen should be guided by epidemiological data and the patient's medical history. In Korea, malaria from P. vivax infection has reemerged recently and shows a high transmission rate. When P. vivax-associated HLH is confirmed, administration of antimalarial drugs can result in the resolution of clinical symptoms and cytopenias without end organ damage.
Figures and Tables
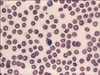
FIG. 1
Peripheral blood smear of Plasmodium vivax-associated hemophagocytic lymphohistiocytosis. Double infection of a single cell with trophozoite and mature macrogametocyte is noted. Also, thrombocytopenia with mild normocytic normochromic anemia is observed (Wright stain, ×1,000).
References
1. Rouphael NG, Talati NJ, Vaughan C, Cunningham K, Moreira R, Gould C. Infections associated with haemophagocytic syndrome. Lancet Infect Dis. 2007. 7:814–822.

2. Henter JI, Aricò M, Elinder G, Imashuku S, Janka G. Familial hemophagocytic lymphohistiocytosis. Primary hemophagocytic lymphohistiocytosis. Hematol Oncol Clin North Am. 1998. 12:417–433.
3. Henter JI, Horne A, Aricò M, Egeler RM, Filipovich AH, Imashuku S, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007. 48:124–131.

4. Aouba A, Noguera ME, Clauvel JP, Quint L. Haemophagocytic syndrome associated with Plasmodium vivax infection. Br J Haematol. 2000. 108:832–833.
5. Park TS, Oh SH, Choi JC, Kim HH, Chang CL, Son HC, et al. Plasmodium vivax malaria complicated by hemophagocytic syndrome in an immunocompetent serviceman. Am J Hematol. 2003. 74:127–130.

6. Bae E, Jang S, Park CJ, Chi HS. Plasmodium vivax malaria-associated hemophagocytic lymphohistiocytosis in a young man with pancytopenia and fever. Ann Hematol. 2011. 90:491–492.

7. Singh ZN, Rakheja D, Yadav TP, Shome DK. Infection-associated haemophagocytosis: the tropical spectrum. Clin Lab Haematol. 2005. 27:312–315.

8. Pahwa R, Singh T, Khurana N. Hemophagocytic syndrome in malaria and kala-azar. Indian J Pathol Microbiol. 2004. 47:348–350.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download