Abstract
This study was designed to investigate the effects an 8-Br-cGMP on the neuronal activity of rat vestibular nuclear cells. Sprague-Dawley rats aged 14 to 16 days were decapitated under ether anesthesia. After treatment with pronase and thermolysin, the dissociated vestibular nuclear cells were transferred into a chamber on an inverted microscope. Spontaneous action potentials and potassium currents were recorded by standard patch-clamp techniques under current and voltage-clamp modes. Twelve vestibular nuclear cells revealed excitatory responses to 1-5 µM of 8-Br-cGMP, and 3 neurons did not respond to 8-Br-cGMP. Whole potassium currents of vestibular nuclear cells were decreased by 8-Br-cGMP (n=12). After calcium-dependent potassium currents were blocked by tetraethylammonium, the potassium currents were not decreased by 8-Br-cGMP. These experimental results suggest that 8-Br-cGMP changes the neuronal activity of vestibular nuclear cells by blocking the calcium-dependent potassium currents that underlie the afterhyperpolarization.
The vestibular nuclear complex in the brainstem is composed of 4 major nuclei: superior, inferior, medial, and lateral. These nuclei are involved in the control of body balance and equilibrium by integrating various sensory information from semicircular canals, otolith organs, vestibulocerebellum, peripheral proprioceptors, and vision.1
The processing of sensory information in the vestibular nuclear complex is related to many neurotransmitters, such as glutamate from primary vestibular neurons, GABA from contralateral vestibular nuclei and vestibulocerebellum, and noradrenaline from locus coeruleus, glycine, and opioids.2,3 These transmitters regulate the vestibular reflexes by increasing or decreasing the neuronal excitabilities of vestibular nuclear neurons that fire spontaneously.4,5
Podda et al reported two types of cyclic nucleotide-gated channels, the olfactory-type and the rod-type cyclic nucleotide-gated channels in medial vestibular nuclear neurons, which are found in olfactory sensory cells and rod photoreceptor cells, respectively.6 Despite some reports on the existence of cyclic nucleotide-gated channels in the central nervous system, there are only a few reports concerning the effects of the cyclic nucleotide-gated channels on excitability in vestibular nuclear neurons. This study aimed to investigate the effects of 8-Br-cGMP by monitoring the changes in spontaneous firing patterns and potassium currents of medial vestibular nuclear neurons using patch-clamping experiments and the possible action of 8-Br-cGMP as a facilitating factor of vestibular compensation.
The Institutional Committee of Laboratory Animal Care and Use approved the experimental protocol. Coronal slices of the brainstem of Sprague-Dawley rats aged 14 to 17 days were prepared as described previously for rats.7 Briefly, the animals were anesthetized with ether and decapitated. The brainstem was rapidly removed into ice-cold artificial cerebrospinal fluid. Coronal slices (400-µm-thick) of the brainstem were made with a sliding microtome (Vibroslice; WPI, Sarasota, FL, USA). The slices were incubated in artificial cerebrospinal fluid well saturated with 95% O2/5% CO2 at room temperature for 1 h. The slices were treated with pronase (0.2 mg/ml) for 40 to 60 min and subsequently exposed to thermolysin (0.2 mg/ml) for 10 min at 32℃. After the enzyme digestion, a portion of the medial vestibular nuclear neurons was removed by micropunching and gently agitated. The dissociated neurons were transferred into a recording chamber mounted on an inverted microscope (IX 70; Olympus, Tokyo, Japan).
The whole-cell membrane potentials were recorded at room temperature by using standard patch-clamp techniques. The patch pipette had a resistance of 3 to 6 MΩ when filled with a pipette solution. Membrane potentials were measured with an Axopatch 200B voltage-clamp amplifier (Axon Instrument, Foster City, CA, USA). Command pulses were applied by using an IBM-compatible computer and pCLAMP 7 software (Axon Instrument). The data were filtered at 5 kHz and displayed on an oscilloscope (Tektronik, Wilsonville, OR, USA), a computer monitor, and a pen recorder (Grass Polygraph, Quincy, MA, USA).
The artificial cerebrospinal fluid had the following composition (mM): NaCl 124, KCl 5, KH2PO4 1.2, MgSO4 1.3, CaCl2 2.4, D-glucose 10, NaHCO3 24. The external solution for recordings had the following composition in mM: NaCl 124, KCl 5, MgSO4 1.3, NaHCO3 26, CaCl2 2, NaH2PO4 1, glucose 11 (pH 7.4 with KOH). The internal solution (the patch pipette solution) had the following composition in mM: K-gluconate 122.5, KCl 17.5, NaCl 8, HEPES 10, EGTA 0.5, Mg-ATP 4 (pH 7.3 with KOH).
The drugs were made from stock solutions that were made up in distilled water and diluted to the desired concentration in external solution. The drugs were applied to the medial vestibular nuclear cells by switching the perfusion inlet tube to the bath chamber. They were purchased from Sigma Chemical Co. (St. Louis, MO, USA). The average firing rate and membrane potential were calculated in recordings over 10 minutes. The resting membrane potential was measured at the lowest point of the rising phase of the spike. The afterhyperpolarization amplitude of the action potential was measured as the difference in membrane potential between the spike threshold and the minimum post-falling phase of the spike.
Current clamp mode whole-cell patch clamp recordings were performed on the medial vestibular nuclear neurons to investigate direct effects of 8-Br-cGMP on the spontaneous activity of the medial vestibular nuclear neurons. Medial vestibular nuclear neurons isolated from rat brainstem have round or pyramidal shaped cell bodies. When the command current was fixed to 0 nA, the neurons revealed spontaneous firing action potentials, a typical electrical characteristic of medial vestibular nuclear neurons, ranging from 3.5 to 5 spikes/s.
The medial vestibular nuclear neurons (n=12) responding to 8-Br-cGMP revealed a uniform pattern response. Three medial vestibular nuclear neurons did not respond to 8-Br-cGMP. The spike frequency was increased from 3.91±0.21 spikes/s to 5.47±0.79 spikes/s (p<0.05) and 6.81±0.36 spikes/s (p<0.05) by 1 µM and 5 µM 8-Br-cGMP, respectively. The resting membrane potential was increased from -42.23±0.31 mV to -41.02±0.12 mV and -40.01±0.43 mV by 1 µM and 5 µM 8-Br-cGMP, respectively. The depth of after-hyperpolarization was decreased to 11.11±0.21 mV and 9.12±0.32 mV (p<0.05) from 11.87±0.23 mV by 1 and 5 µM CGS-12066A, respectively (Fig. 1).
To explore which currents are related to the modification of spontaneous firing action potentials of the medial vestibular nuclear neurons by 8-Br-cGMP, changes in outward potassium currents by 8-Br-cGMP were studied under the voltage clamp mode. The potassium currents were activated by 400-ms test pulses from -60 to +40 mV in 10-mV increments from a holding potential of -70 mV.
The 8-Br-cGMP at concentrations of 1 µM and 5 µM applied to the bath decreased the potassium currents of the 12 cells tested and did not change the potassium currents of 1 cell. The mean peak current of the medial vestibular nuclear neurons was 2723±037 pA in the control. The potassium currents of the vestibular nuclear neurons treated with 8-Br-cGMP were decreased to 2,231±123 pA (p<0.05) and 1,321±099 pA (p<0.05) by 1 and 5 µM 8-Br-cGMP, respectively (Fig. 2).
Medial vestibular nuclear neurons are known to possess A-type potassium currents, delayed rectifier potassium currents, and calcium-dependent potassium currents. To identify the potassium currents affected by 8-Br-cGMP, we blocked the calcium-dependent potassium currents and tested the effects of 8-Br-cGMP. For blocking the calcium-dependent potassium currents, 0.1 mM tetraethylammonium was used. After blockade of the calcium-dependent potassium currents, the peak current of the medial vestibular nuclear neurons (n=20) was reduced to 1241±321 pA (p<0.05) from 1931±214 pA, and 8-Br-cGMP did not affect the outward potassium currents (1211±721 pA) (Fig. 3).
Cyclic nucleotides are known to be major secondary messengers in neuronal cells and they act on cyclic nucleotide-gated channels directly or via activation of protein kinase. Cyclic nucleotide-gated channels (CNG) activated by ligands are heterodimers composed of alpha and beta subunits and were first identified in rod cells (CNG1), olfactory receptors (CNG2), and cone cells (CNG3).8-12 Cyclic nucleotide-gated channels are distributed widely in brain, especially the hippocampus, cerebral cortex, and cerebellum. Among the cyclic nucleotide-gated channels, cyclic nucleotide-gated channel l and channel 3 are activated by cGMP, whereas channel 2 is activated by cAMP and cGMP with the same affinity.13,14
In the present study, we performed a whole-cell patch clamp to explore cGMP receptor-mediated effects on acutely isolated rat medial vestibular nuclear neurons without neuronal connections with the other structures in central nervous system. 8-Br-cGMP depolarized the medial vestibular nuclear neurons and increased the spike frequency. It inhibited whole potassium currents, and after blockade of calcium-dependent potassium currents by tetraethylammonium, 8-Br-cGMP did not inhibit the potassium currents. These findings suggest that 8-Br-cGMP affects the neuronal activity of the medial vestibular nuclear neurons through calcium-dependent potassium channels.
Potassium channels play an important role in regulating membrane potential and cell excitability by modifying action potential and firing rates.13 Generally, the closure of potassium channels causes depolarization, which increases cell excitability, and the opening of potassium channels produces hyperpolarization, which decreases the excitability.14 Two types of calcium-dependent potassium channels are identified in neuronal cells: SKca and BKca. Activation of BKca channels contributes to action potential repolarization, and activation of SKca channels underlies the after-hyperpolarization.15 As shown in this study, 8-Br-cGMP modified the afterhyperpolarization of medial vestibular nuclear neurons, which is similar to the effects of apamine, a blocker of calcium-dependent potassium currents.16
Vestibular compensation is a process of partial behavioral recovery that occurs following lesions to the vestibular inner ear.17 After unilateral labyrinthectomy, the resting activity in the ipsilateral vestibular nucleus complex markedly decreases, resulting in an imbalance in neuronal activity between the ipsilateral and contralateral vestibular nucleus complexes.18 This imbalance causes severe postural and oculomotor disturbances. Despite permanent loss of the ipsilateral vestibular peripheral inputs, many but not all of these symptoms gradually disappear along with the recovery of resting activity in the ipsilateral medial vestibular nuclear neurons.19
The medial vestibular nucleus is the largest among the vestibular nuclei, where major post-lesional changes occur with the vestibulocerebellum after unilateral labyrinthectomy.20 In the present study, 8-Br-cGMP increased the neuronal excitability of medial vestibular nuclear neurons. Therefore, 8-Br-cGMP may be a good candidate for recovery of the decreased activity of the ipsilateral medial vestibular nucleus by increasing the neuronal excitability of medial vestibular nuclear neurons, which facilitates vestibular compensation.
Figures and Tables
FIG. 1
Excitatory effects of 8-Br-cGMP on spontaneous activity of rat medial vestibular nuclear neurons. 8-Br-cGMP increased the firing rate and decreased the membrane potential and the amplitude of afterhyperpolarization.
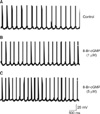
FIG. 2
Effects of 8-Br-cGMP on the outward potassium currents in medial vestibular nuclear neurons. In each panel, the cell was held at -70 mV and test depolarizations with durations of 400 ms were applied from -60 mV to +40 mV in 10-mV increments. The 8-Br-cGMP decreased the outward potassium currents in a dose-dependent manner.

ACKNOWLEDGEMENTS
This work was supported by the Chonnam Hospital Research Institute of Clinical Medicine (CUHRI-2009).
References
1. Darlington CL, Flohr H, Smith PF. Molecular mechanisms of brainstem plasticity. The vestibular compensation model. Mol Neurobiol. 1991. 5:355–368.
2. Smith PF, Darlington CL. Neurochemical mechanisms of recovery from peripheral vestibular lesions (vestibular compensation). Brain Res Brain Res Rev. 1991. 16:117–133.

3. Zennou-Azogui Y, Borel L, Lacour M, Ez-Zaher L, Ouaknine M. Recovery of head postural control following unilateral vestibular neurectomy in the cat. Neck muscle activity and neuronal correlates in Deiters' nuclei. Acta Otolaryngol Suppl. 1993. 509:1–19.

4. Yamanaka T, Sasa M, Amano T, Miyahara H, Matsunaga T. Role of glucocorticoid in vestibular compensation in relation to activation of vestibular nucleus neurons. Acta Otolaryngol Suppl. 1995. 519:168–172.

5. Vibert N, Bantikyan A, Babalian A, Serafin M, Mühlethaler M, Vidal PP. Post-lesional plasticity in the central nervous system of the guinea-pig: a "top-down" adaptation process? Neuroscience. 1999. 94:1–5.

6. Podda MV, Marcocci ME, Oggiano L, D'Ascenzo M, Tolu E, Palamara AT, et al. Nitric oxide increases the spontaneous firing rate of rat medial vestibular nucleus neurons in vitro via a cyclic GMP-mediated PKG-independent mechanism. Eur J Neurosci. 2004. 20:2124–2132.

7. Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981. 391:85–100.

8. Ahmad I, Leinders-Zufall T, Kocsis JD, Shepherd GM, Zufall F, Barnstable CJ. Retinal ganglion cells express a cGMP-gated cation conductance activatable by nitric oxide donors. Neuron. 1994. 12:155–165.

9. Goulding EH, Tibbs GR, Siegelbaum SA. Molecular mechanism of cyclic-nucleotide-gated channel activation. Nature. 1994. 372:369–374.

10. Wei JY, Cohen ED, Genieser HG, Barnstable CJ. Substituted cGMP analogs can act as selective agonists of the rod photoreceptor cGMP-gated cation channel. J Mol Neurosci. 1998. 10:53–64.

11. Wei JY, Roy DS, Leconte L, Barnstable CJ. Molecular and pharmacological analysis of cyclic nucleotide-gated channel function in the central nervous system. Prog Neurobiol. 1998. 56:37–64.

13. Kingston PA, Zufall F, Barnstable CJ. Widespread expression of olfactory cyclic nucleotide-gated channel genes in rat brain: implications for neuronal signalling. Synapse. 1999. 32:1–12.

14. Barnstable CJ, Wei JY, Han MH. Modulation of synaptic function by cGMP and cGMP-gated cation channels. Neurochem Int. 2004. 45:875–884.

15. Johnston AR, MacLeod NK, Dutia MB. Ionic conductances contributing to spike repolarization and after-potentials in rat medial vestibular nucleus neurones. J Physiol. 1994. 481:61–77.

16. Kaplan DR, Miller FD. Neurotrophin signal transduction in the nervous system. Curr Opin Neurobiol. 2000. 10:381–391.

17. Ris L, Godaux E. Neuronal activity in the vestibular nuclei after contralateral or bilateral labyrinthectomy in the alert guinea pig. J Neurophysiol. 1998. 80:2352–2367.

18. Sansom AJ, Darlington CL, Smith PF. Pretreatment with MK-801 reduces spontaneous nystagmus following unilateral labyrinthectomy. Eur J Pharmacol. 1992. 220:123–129.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download