Abstract
Metastasis to the thyroid gland from distant cancer is rare, and, in some cases, is a diagnostic challenge. Here, we report a case of metastatic renal cell carcinoma of the thyroid gland. A 77-year-old man presented with a neck mass detected about 1 month previously. He had undergone a right nephrectomy owing to renal cell carcinoma 14 years previously. Fine needle aspiration cytology showed a few atypical follicular cells with nuclear atypia. Under a tentative diagnosis of papillary thyroid carcinoma, a total thyroidectomy was performed. The histologic and immunohistochemical studies of the surgical specimens indicated that the thyroid masses were metastatic renal cell carcinoma to the thyroid.
Although clinically recognized metastatic thyroid cancer is rare, data from autopsy show that thyroid metastasis findings range from 1.9% to 24.2%, with the common sources of primary cancer being skin, lung, and breast.1,2 However, of the clinically recognized metastases to the thyroid, more than 50% of the time the primary cancer is renal cell carcinoma (RCC).3 RCC represents 3% of all adult malignancies.4 The presentation and behavior of thyroid metastases from renal cancer are variable.5 Here, we report a very unusual case of metastatic RCC to the thyroid gland in a patient who had undergone right nephrectomy for RCC 14 years earlier.
A 77-year-old man was referred to our department from another hospital for thyroid masses detected about 1 month previously. He had no subjective symptoms, such as stridor, dyspnea, hoarseness, or dysphagia. His medical history was remarkable in that he had undergone right nephrectomy for stage I RCC 14 years previously. The pathologic type of RCC was revealed as clear cell. He had type 2 diabetes mellitus and essential hypertension for the past 5 years. Medications for chronic diseases as prescribed by his primary care physician were as follows: felodipine 5 mg, losartan 50 mg/hydrochlorothiazide 12.5 mg, glimepiride 2 mg, and metformin 750 mg, each daily. A soft and painless 7.0×5.0 cm sized mass was palpable on the left thyroid, and a smaller 3.5 cm soft mass was palpable on the right thyroid. Fine needle aspiration (FNA) cytology showed a few atypical follicular cells with nuclear atypia, suggesting papillary thyroid carcinoma. A computed tomography (CT) image of the neck showed two large cystic and degenerating thyroid masses: a 7.2×5.5 cm sized mass on the left thyroid and a 3.8×3.8 cm sized mass on the right thyroid without lymph node (LN) metastasis (Fig. 1). He had euthyroidism and all of his laboratory data were normal. All of these results led the patient to undergo total thyroidectomy. No apparent extrathyroidal invasion or enlarged LNs were observed during the operation. On gross inspection of the resected specimen, two large, well-circumscribed, mixed cystic and solid masses were found, one in each thyroid lobe, with hemorrhagic changes in the left mass (Fig. 2A). The histopathologic findings of the thyroid masses were small nests of clear cells separated by a prominent sinusoidal network (Fig. 2B) with abundant optically empty cytoplasm, sharply outlined boundaries, and moderately atypical nuclei (Fig. 2C). These microscopic findings were similar to those of the previous renal cell carcinoma, clear cell type (Fig. 2D). Immunohistochemical (IHC) studies were performed for thyroglobulin (Tg), thyroid transcription factor-1 (TTF-1), CD10, cytokeratin, vimentin, and epithelial membrane antigen (EMA). The clear cells displayed strong immunoreactivity for cytokeratin, vimentin, CD10, and EMA and were negative for Tg and TTF-1 (Fig. 3). Taken together, these microscopic and IHC findings confirmed that the thyroid masses were metastatic RCC, clear cell type. An abdominal CT scan revealed a 3.7 cm sized mass on the right nephrectomy site with a normal left kidney. A laparotomy was recommended for the patient. However, he refused the surgery and further medical therapy for RCC.
Metastatic carcinomas to the thyroid gland are rarely found in clinical practice.5 RCC is well-known for its highly variable clinical presentations and courses, being called the "internist's tumor".6 Rarely, a thyroid mass can be an initial clinical sign of relapse of RCC after a surgical treatment or can even appear as an initial clinical presentation of RCC before the detection of primary cancer.5 Possible reasons for the low frequency of clinical metastatic thyroid carcinoma are not known, although some explanations have been proposed: rich blood supply, high oxygen tension, and high iodine content in the thyroid.7
In the absence of a detailed clinical history, the initial presentation of a thyroid nodule in an otherwise healthy subject makes the diagnosis of metastatic disease challenging. This is complicated further by histologic similarities between metastatic foci and primary thyroid tumors.5 There are no specific clinical features attributable to metastases to the thyroid.3,5 Our patient had no subjective symptoms associated with the thyroid masses, and his thyroid function was normal. Also, the initial FNA cytology suggested primary papillary carcinoma of the thyroid.
The true metastatic nature of the tumor is usually recognized after tumor sampling with pathologic assessment. Distinguishing primary from metastatic thyroid tumors is usually performed with Tg antibody IHC staining. The use of negative TTF-1 staining or positive periodic acid-Schiff staining in conjunction with negative Tg staining can accurately lead to the diagnosis of metastatic disease.8,9 The majority of RCCs are immunoreactive to keratin and EMA.5,7,10 The microscopic examinations of the resected lesions in our patient showed small nests of clear cells separated by a prominent sinusoidal network with abundant optically empty cytoplasm. The clear cells were immunohistochemically positive for cytokeratin, vimentin, CD10, and EMA and were negative for Tg and TTF-1. These microscopic and IHC findings were consistent with those of metastatic RCC to the thyroid in our patient.
About 20% to 30% of cases of RCC patients experience cancer relapse distantly after curative nephrectomy.6 Furthermore, unusual sites of metastases are characteristic of renal cancer, and virtually any organ site can be involved, including the thyroid.6 Although there have been reports of RCC metastasis to the thyroid, and although it is well-known that RCC has variable natural histories, this was a very unusual case of metastatic RCC in the thyroid gland detected 14 years after curative nephrectomy. The mean time before a relapse in the thyroid gland after initial nephrectomy was previously reported to be 9.4 years.5
In conclusion, our case experience and literature review suggest that long-term follow-up including physical examination of the thyroid gland is required after initial nephrectomy for patients with RCC.
Figures and Tables
FIG. 1
CT scan of the neck showed two large cystic and degenerating thyroid masses. The mass on the left thyroid gland was a 7.2×5.5 cm sized mass with severe necrosis. The mass on right thyroid was a 3.8×3.8 cm sized well-circumscribed mass. There was no lymph node metastasis. (A) Axial view. (B) Coronal view.
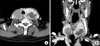
FIG. 2
Macro- and microscopic findings of renal cell carcinoma metastasis in the thyroid. (A) On gross inspection of the resected specimen, there were well demarcated masses with cystic change and hemorrhage in both lobes of the thyroid gland. Characteristic histologic appearance is shown with small nests of clear cells separated by a prominent sinusoidal network (B, H&E stain, ×100) with abundant optically empty cytoplasm, sharply outlined boundaries, and moderately atypical nuclei (C, H&E stain, ×200). These microscopic findings were identical to those of the previously resected primary renal cell carcinoma, clear cell type (D, H&E stain, ×200).
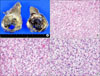
FIG. 3
Immunohistochemical staining of renal cell carcinoma metastasis to the thyroid. The tumor cells characteristically expressed cytokeratin (A), vimentin (B), CD10 (C), and epithelial membrane antigen (D), but did not express thyroglobulin (E) or thyroid transcription factor-1 (F) (avidin-biotin-peroxidase, ×200).

References
1. Willis RA. Metastatic tumours in the thyreoid gland. Am J Pathol. 1931. 7:187–208.3.
2. Lasser A, Rothman JG, Calamia VJ. Renal-cell carcinoma metastatic to the thyroid. Aspiration cytology and histologic findings. Acta Cytol. 1985. 29:856–858.
3. Chen H, Nicol TL, Udelsman R. Clinically significant, isolated metastatic disease to the thyroid gland. World J Surg. 1999. 23:177–180.

4. Palazzo FF, Bradpiece HA, Morgan MW. Renal cell carcinoma metastasizing to the thyroid gland. Scand J Urol Nephrol. 1999. 33:202–204.

5. Heffess CS, Wenig BM, Thompson LD. Metastatic renal cell carcinoma to the thyroid gland: a clinicopathologic study of 36 cases. Cancer. 2002. 95:1869–1878.

7. Linton RR, Barney JD, Moorman HD, Lerman J. Metastatic hypernephroma of the thyroid gland. Surg Gynecol Obstet. 1946. 83:493–498.
8. Shimizu K, Nagahama M, Kitamura Y, Chin K, Kitagawa W, Shibuya T, et al. Clinicopathological study of clear-cell tumors of the thyroid: an evaluation of 22 cases. Surg Today. 1995. 25:1015–1022.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download