INTRODUCTION
Tea, such as black tea, green tea, or oolong tea, derived from the leaves of Camellia sinensis, is one of the most popularly consumed beverages worldwide. The differences between black, green, and oolong tea lie in their fermentation process. Whereas black tea is fully fermented, green tea is unfermented, and oolong tea is partially fermented. Tea contains many compounds such as polyphenols, polysaccharides, amino acids, and vitamins. Among the compounds found in tea, polyphenols are well-known to have antioxidant, anti-inflammatory, and immunomodulatory effects, as well as anti-tumor, anti-carcinogenic, and anti-mutagenic activities in various diseases, including cancers, cardiovascular diseases, and intestinal inflammation.1-3
Theaflavins are the major polyphenol in black tea. Theaflavins are categorized into the following forms: theaflavin, theaflavin-3-gallate, theaflavin-3'-gallate, and theaflavin-3,3'-gallate. Theaflavins have been shown to possess potent antioxidant and anti-inflammatory activities in in vitro and in vivo studies.4-9 However, the underlying mechanisms responsible for the health benefits of black tea polyphenols are largely unknown compared with those of green tea.
Ulcerative colitis and Crohn's disease are the two major manifestations of inflammatory bowel disease (IBD), encompassing chronic inflammatory conditions of the gastrointestinal tract. Their etiologies remain unknown, but evidence points to genetic changes that cause dysregulated host immune responses directed against luminal or epithelial antigens or triggered by other environmental factors. The activation of the immune system induces the infiltration and migration of immune cells into lesion sites and is followed by the production of pro-inflammatory cytokines. Among the immune cells, mucosal macrophages play a major role in chronic intestinal inflammation. The sustained production of pro-inflammatory cytokines leads to the induction of chemokines and cell adhesion molecules in intestinal inflammation.10
Interleukin-6 (IL-6) is considered to play an important role in the transition between acute and chronic inflammation, as well as the transition between innate and acquired immunity, especially in intestinal inflammation. It modulates chemokine and cell adhesion molecule expression and apoptosis, suppressing neutrophil infiltration and promoting the accumulation of mononuclear leukocytes, which directs the resolution of acute inflammation and the activation of acquired immunity.11,12 Monocyte chemoattractant protein (MCP-1) is the most important chemokine that regulates the migration and infiltration of monocytes and macrophages and works as a key factor in initiating the various inflammatory responses.13 Intercellular adhesion molecule-1 (ICAM-1) is a surface receptor expressed primarily by endothelial cells that captures circulating leukocytes and mediators across the endothelium. ICAM-1 is also involved in several subsequent steps of the inflammatory cascade, including IBD.14
The production of pro-inflammatory mediators including inflammatory cytokines, chemokines, and cell adhesion molecules is controlled by the activity of transcription factors, such as the transcriptional factor nuclear factor-κB (NF-κB) and mitogen-activated protein kinase (MAPK).15 Thus, the activation of NF-κB and MAPKs is known to be extremely important in the pathogenesis of IBD and has been proposed as a major culprit and therapeutic target for IBD.16-19
Therefore, in the present study, we investigated the impact of theaflavin on lipopolysaccharide (LPS)-induced NF-κB and MAPK signaling pathways and the expression of pro-inflammatory mediators in bone marrow-derived macrophages (BMMs) isolated from mice.
MATERIALS AND METHODS
1. Reagents
Theaflavin (purity >80%) was obtained from Sigma-Aldrich (St. Louis, MO, USA) and was dissolved in dimethyl sulfoxide (DMSO; Sigma). LPS from Escherichia coli (serotype 0111:B4) was also purchased from Sigma-Aldrich and was dissolved in sterile, pyrogen-free phosphate-buffered saline (PBS). PD98059 (extracellular signal-regulated kinase [ERK] inhibitor), SP600125 (c-Jun-N-terminal kinase [JNK] inhibitor), SB203580 (p38 MAPK inhibitor), and Bay11-7082 (NF-κB inhibitor) were purchased from Calbiochem (San Diego, CA, USA). These inhibitors were dissolved in DMSO.
2. Cell culture
Bone marrow cells were isolated from 5- to 8-week-old ICR mice as previously described.19 Mice were killed by cervical dislocation. Femur and tibia were aseptically removed and dissected free of adherent soft tissue. The bone ends were cut, and the marrow cavity was flushed out into a petri dish by slowly injecting MEM-α medium (Hyclone, Loan, UT, USA) at one end of the bone with a sterile 21-gauge needle. The bone marrow suspension was carefully agitated with a plastic Pasteur pipette to obtain a single-cell suspension. Bone marrow cells were washed and depleted of red blood cells (RBCs) by hypotonic lysis with RBC lysing buffer (Sigma). After washing twice with PBS, the cells were suspended in MEM-α medium supplemented with 10% fetal bovine serum and 50 units/ml penicillin and 50 µg/ml streptomycin (Gibco Laboratories, Grand Island, NY, USA). The number of viable cells was determined with trypan blue (Gibco) and bone marrow cells were cultured on 10 cm2 tissue culture dishes in total amounts of 2×106 cells/dish. A total of 10 ng/ml of mouse macrophage colony stimulating factor (M-CSF; BioSource, Camarillo, CA, USA) was added to every 10-cm dish to differentiate BMMs. On day 3, nonadherent cells were discarded and adherent cells (immature BMMs) were suspended in fresh MEM-α with M-CSF and used in subsequent experiments. All of the cells were cultured at 37℃ under a humidified atmosphere containing 5% CO2. For dose- and time-dependent studies, the cells were treated with various concentrations of test compounds for the indicated times with or without LPS. Cells that were used as controls were treated with the vehicle (DMSO) only for similar durations.
3. Cell viability
To assess cell viability, cells were seeded at a density of 1×104 cells/well in 96-well culture plates, treated with indicated concentrations of theaflavin and LPS, and incubated for 24 h. Cell viability was determined by use of the EZ-CyTox (tetrazolium salts, WST-1) cell viability assay kit (Daeil Lab Inc, Seoul, Korea). After application of WST-1 reagent for 1 to 2 h at 37℃, cell viability was measured with a microplate reader (Infinite M200, Tecan, Austria GmbH, Austria) with Magellan V6 data analysis software (Tecan). Triplicate wells were used for each experiment and all experiments were conducted in at least triplicate.
4. RT-PCR
Total RNA was extracted by using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and following the standard protocol. The quantity and purity of total RNA were measured by using a Nanodrop reader. Subsequently, 1 µg total RNA was converted to first-strand cDNA by using MMLV reverse transcriptase (Invitrogen) and RNAsin (Takara, Otsu, Shiga, Japan). cDNA was amplified by using gene-specific primers and Go taq Polymerase (Promega, Madison, WI, USA). Primer sequences were as follows: IL-6, 5'-GGATACCACCCACAACAGACC-3'/5'-GGTCCTTAGCCACTCCTTCTG-3'; MCP-1, 5'-GAAGACCTTAGGGCAGATGCAG-3'/5'-CTGTCATGCTTCTGGGCCTG-3'; ICAM-1, 5'-GACCCCAAGGAGATCACATTCAC-3'/5'-GTCCAGTTCCCCAAGCAGTCC-3'; GAPDH, 5'-ACCACAGTCCATGCCATCAC-3'/5'-TCCACCACCCTGTTGCTGTA-3'. GAPDH was used as an internal control.
5. Western blotting
The cells were exposed to LPS (1 µg/ml) in the absence or presence of pretreatment with 50-100 µM theaflavin. Then after 10 or 30 min of incubation at 37℃, the cells were washed twice with cold PBS and lysed with RIPA buffer (1 M Tris-HCl, 150 mM NaCl, 1% Triton X-100, 2 mM EDTA) with 1 mM PMSF, Halt™ Phosphatase inhibitor, and HaltTM Protease inhibitor cocktail (Thermo, Rockford, IL, USA) for 15 min at 4℃. The resolved proteins underwent centrifugation at 15,000×g for 20 min at 4℃. The protein quantification of the resulting supernatants was determined by using BCA™ protein assay (Thermo), and all samples were adjusted to equal protein content before analysis. The protein samples were separated by 12% SDS-PAGE (sodium dodecyl sulfate polyacrylamide gel electrophoresis), and the separated proteins were transferred electrophoretically from the gel to the surface of PVDF membranes (Millipore, Billerica, MA, USA). The analysis used primary antibodies as described by the manufacturer of the antibodies; polyclonal anti-IκBα, phospho-IκBα, ERK1/2, phospho-ERK1/2, JNK, phospho-JNK, p38, phospho-p38 (Cell Signaling, Danvers, MA, USA), and polyclonal anti-GAPDH (Santa Cruz Biotechnology, Santa Cruz, CA, USA). After final rinsing with TBST, the membrane was incubated with secondary HRP-linked anti-rabbit IgG for 1 h. After washing, the immunoblots were visualized by use of chemiluminescence (ECL) HRP substrate (Millipore) and were analyzed with an image analyzer (Ras-4,000, Fujifilm, Tokyo, Japan).
6. Immunofluorescence
The cells were plated on 8-chamber slides (Nunc, Rochester, NY, USA) and were exposed to LPS (1 µg/ml) for 30 min in the absence or presence of pretreatment with 50 µM theaflavin. Then the cells were rinsed in PBS and fixed by incubation with 4% formaldehyde in PBS for 15 min at room temperature. After a further wash with PBS, cells were permeabilized with PBS containing 0.25% Triton X-100. Cells were blocked in PBS containing 1% BSA and 10% goat serum. The cells were incubated with polyclonal anti-RelA antibody (Santa Cruz) in primary antibody dilution buffer overnight at 4℃. After washing with PBS, Alexa 488 (green) coupled secondary antibody (Invitrogen) was applied for 15 min at room temperature. Coverslips were mounted to slides by using ProLong Gold antifade reagent containing DAPI (Invitrogen) and were photographed by use of fluorescent microscopy.
RESULTS
1. Effect of theaflavin on cell viability
To examine the effect of theaflavin on cell viability, BMMs were preincubated for 24 h with the indicated concentrations of theaflavin or LPS. As shown in Fig. 1, no significant cytotoxicity of theaflavin or LPS was observed under the experimental conditions.
2. Effect of theaflavin on LPS-induced expression of pro-Inflammatory mediators
The inhibition of pro-inflammatory mediators in LPS-stimulated BMMs by theaflavin was investigated. The cells were pretreated with 50 µg/ml theaflavin for 1 h before stimulation with 1 µg/ml LPS for 1 or 4 h. Theaflavin significantly reduced the LPS-induced accumulation of IL-6, MCP-1, and ICAM-1 mRNA as determined by RT-PCR (Fig. 2).
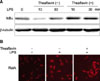
3. Effect of theaflavin on LPS-induced NF-κB activation
NF-κB regulates the expression of pro-inflammatory mediators that play a key role in inflammatory-related injury. To determine the inhibitory action of theaflavin on the expression of pro-inflammatory mediators, we examined the cytoplasmic levels of IκBα proteins by Western blotting. After stimulation of BMMs with LPS for 10 or 30 min, the cytosolic IκBα protein was significantly degraded. The LPS-induced IκBα degradation was dramatically inhibited by co-incubation of LPS plus theaflavin (Fig. 3A). The nuclear translocation of RelA follows IκBα degradation. Therefore, we tested whether theaflavin perturbed the distribution of RelA as assessed by nuclear accumulation. As shown in Fig. 3B, co-incubation with LPS plus theaflavin inhibited the nuclear translocation of RelA. These results suggest that inhibition of pro-inflammatory mediator production by theaflavin occurred via blocking of NF-κB signaling.
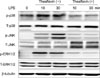
4. Effect of theaflavin on LPS-induced phosphorylation of MAPK signal proteins
It is well known that the expression of pro-inflammatory mediators is regulated by the activation of MAPK pathways such as ERK, JNK, and p38 MAPK. Accordingly, we examined the effect of theaflavin on LPS-induced MAPK signaling in BMMs by Western blotting. Cells were pretreated with specified concentrations of theaflavin for 1 h and stimulated with LPS (1 µg/ml) for various times. Theaflavin pretreatment resulted in a decrease in the level of phosphorylated ERK1/2, JNK, and p38 MAPK in BMMs (Fig. 4).
5. Effect of MAPK and NF-κB inhibitors on LPS-induced expression of pro-inflammatory mediators
We examined the activation of MAPK and NF-κB signaling as involved in the regulation of LPS-induced expression of pro-inflammatory mediators. Inhibitors of NF-κB, ERK, JNK, and p38 MAPK signaling pathways were used to determine which of these pathways were involved in theaflavin action. Bay11-7082 (NF-κB inhibitor) completely abrogated the LPS-induced expression of pro-inflammatory mediators (Fig. 5A). Also, the inhibitory effect of theaflavin on IL-6 and ICAM-1 expression was inhibited by SB203580 (p38 MAPK inhibitor). The inhibitory effect of theaflavin on MCP-1 expression was inhibited by SP600125 (JNK inhibitor) (Fig. 5B). These results suggest that the inhibitory effect of theaflavin on the expression of pro-inflammatory mediators is mediated by blockade of the NF-κB and MAPK signaling pathways.
DISCUSSION
Black tea has been shown to elicit antioxidant, anti-carcinogenic, anti-inflammatory, and anti-mutagenic properties, and such properties have been attributed to its polyphenolic compounds. Theaflavins are the most abundant polyphenols present in black tea and are believed to provide the various health benefits observed with black tea consumption. Although black tea is the most widely consumed beverage, the underlying mechanisms of the health-beneficial properties of black tea polyphenols have not yet been thoroughly elucidated as compared with those of green tea.1-3
Theaflavins, which are formed by oxidation of catechins in the leaves of Camellia sinensis during fermentation, have been suggested to reduce oxidative stress and inflammation by their radical-scavenging ability and down-regulation of pro-inflammatory mediators in vitro and in vivo.4-9 Also, theaflavins significantly inhibit proliferation and enhance apoptosis in various cancer cells, resulting in the inhibition of tumorigenesis.3
Although the pathogenesis of IBD remains unsolved, current evidence indicates that IBD causes a dysregulated mucosal immune response in the intestinal wall facilitated by defects in epithelial barrier function and activation of immune cells with excessive production of inflammatory cytokines, chemokines, and cell adhesion molecules, resulting in tissue injury. Among the immune cells, activation of macrophages plays an important role in the initiation, development, and outcome of the immune response in intestinal inflammation.10 However, until now, the effects of theaflavin on macrophages in intestinal inflammation have not been fully investigated. Therefore, in this study, we used BMMs to study the impact of theaflavin on LPS-induced innate signaling and the expression of pro-inflammatory mediators and found out that the mRNA expression of LPS-induced IL-6, MCP-1, and ICAM-1 in BMMs was inhibited by theaflavin treatment. These results suggest that theaflavin possesses anti-inflammatory activity by inhibiting the production of pro-inflammatory mediators and may be beneficial for the treatment of IBD.
NF-κB is a nuclear transcription factor that regulates the expression of genes encoding pro-inflammatory cytokines, chemokines, and cell adhesion molecules that play a key part in inflammation-related injury such as IBD. Inactive NF-κB is present in the cytosol complexed with an inhibitory protein, inhibitor κB (IκB). Ligand binding to particular membrane or cytosolic receptors leads to activation of the key enzyme, IκB kinase (IKK). IKK activation results in phosphorylation of IκB, dissociation of IκB from NF-κB, and ubiquitination and ultimate proteosomal degradation of IκB. Upon activation of NF-κB, it is translocated into the nucleus where it binds to selective target gene promoters and mediates transcriptional activation of numerous genes involved in innate and adaptive immune responses.16,17 Previously, theaflavin inhibited the activation of IKK and subsequent activation of the IκB-α/NF-κB pathway in human lung adenocarcinoma cells, macrophages, and mouse epidermal cells.20-22 Furthermore, theaflavins including theaflavin-3,3'-digallate and thearubigin inhibited nuclear translocation of NF-κB, cytosolic IKK activity, IκBα phosphorylation and degradation, and NF-κB DNA-binding activity in a TNBS-induced colitis model.5,9 In our study, theaflavin inhibited LPS-induced IκBα degradation and nuclear translocation of RelA in BMMs. These results suggest that inhibition of NF-κB by theaflavin is not cell-type specific and that such inhibition operates in vivo. In our study, Bay11-7082, an NF-κB inhibitor, suppressed the LPS-induced accumulation of IL-6, MCP-1, and ICAM-1 mRNA in BMMs. Therefore, our results show that theaflavin inhibits the expression of pro-inflammatory mediators by blockade of NF-κB activation in BMMs.
MAPK signaling pathways play an important role in many physiologic processes, including cell proliferation, differentiation, and apoptosis. Three classes of MAPK are known and include extracellular signal-regulated kinase 1/2 (ERK1/2), c-Jun-N-terminal kinase (JNK), and p38 MAPK. The biological effects of MAPK signaling are mainly executed by the phosphorylation of downstream substrates including transcription factors such as c-Jun and c-Fos.18,19 Accumulated data indicate that activation of MAPKs by mitogen and oxidative stress modulates several different steps in the inflammatory cascade and promotes the production of pro-inflammatory mediators including cytokines, chemokines, and cell adhesion molecules.24-26 Previously, theaflavin treatment induced apoptosis through the down-regulation of phosphorylation of MAPK signaling proteins in human prostatic cancer cells and malignant melanoma cells.27-29 In our study, LPS-induced phosphorylation of ERK1/2, JNK, and p38 MAPK was inhibited by theaflavin. The inhibitory effect of theaflavin on IL-6 and ICAM-1 expression was inhibited by p38 MAPK inhibitor. The inhibitory effect of theaflavin on MCP-1 expression was inhibited by JNK inhibitor. These results suggest that theaflavin negatively modulates the expression of proinflammatory mediators partly dependent on MAPK signaling pathways in BMMs.
In conclusion, theaflavin prevents LPS-induced IL-6, MCP-1, and ICAM-1 expression through blockade of NF-κB and MAPK signaling pathways in BMMs. Therefore, modulation of these signaling pathways by theaflavin may be beneficial in the treatment of IBD.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download