Abstract
We report a neonatal case of “intraluminal” pyloric duplication cyst, causing gastric obstruction after birth. Endoscopy revealed a submucosal cystic lesion approximately 15 mm in size arising from the anterior and inferior surfaces of the pylorus obliterating the pyloric canal. After laparotomy, intraoperative cholangiography was performed, which documented no communication between the cyst and the bilio-pancreatic duct. Gastrotomy was performed transversally over the antrum, and the cyst delivered through the incision. The cyst was incised, the upper part of the cyst wall removed, and a mucosectomy performed on the inner cyst wall of the lower part. The mucosa and muscle of the margin of the cyst were approximated. At follow up of 10 months, the patient is well without any sign of gastric obstruction.
Enteric duplications are rare congenital malformations with only 4% being gastric duplications and the majority being located adjacent to the alimentary tract [1]. “Intraluminal” pyloric duplication is extremely rare [23]. We report a neonate with intraluminal pyloric duplication who presented with gastric outlet obstruction.
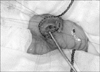
A 3-day-old girl, born by normal vaginal delivery at 38 weeks gestation with a birth weight of 2,718 g, presented with 2 days of non-bilious vomiting and feeding difficulties since birth. A routine prenatal ultrasonography performed at 31 weeks of gestation showed a gastric bubble sign. Repeat ultrasonography at 34 weeks of gestation showed that the gastric bubble sign had disappeared. On presentation, the patient was severely dehydrated and weighed only 2,436 g. Her abdomen was distended. An olive-like mass was palpable in the upper abdomen following insertion of a nasogastric tube. Laboratory test results revealed electrolyte disturbances consistent with severe hypovolemia and alkalosis. There were no vertebral anomalies identified. Plain abdominal radiography demonstrated a distended stomach with no gas distal to the stomach—findings consistent with gastric outlet obstruction. Ultrasonography revealed an “intraluminal” cyst arising from the pyloric orifice and virtually obliterating it (Fig. 1). The wall of the lesion had a hypoechoic outer rim and a thick echogenic inner rim or gastrointestinal signature [4] and the lumen was hypoechoic. A gastrografin swallow with upper gastrointestinal study demonstrated a mass obstructing the pyloric orifice. A small quantity of gastrografin passed around the perimeter of the lesion and entered the duodenum. Following fluid resuscitation and stabilization, an enteral digestive (ED) tube was inserted on day 7. Magnetic resonance cholangiopancreatography (MRCP) demonstrated that the cystic lesion was located in the pyloric orifice. However, T2-weighted sequences, obtained in the coronal plane did not reveal whether the fluid content of the lesion communicated with the bile duct or not. On day 22, body weight was 2,815 g and an operative exploration was undertaken. A laparotomy was performed through a transverse upper abdominal incision. No cyst was identified, but, an elastic mass was palpable at the pyloric orifice. Intraoperative endoscopy revealed a submucosal gastric lesion approximately 15 mm in size arising from the anterior and inferior surfaces of the pylorus (Fig. 2). Intraoperative cholangiography documented no communication between the cystic lesion and the bile or pancreatic ducts. Gastrotomy was performed transversally over the antrum just proximal to the cyst, and the cyst was delivered in toto through the incision. The cyst was incised (Fig. 3) and a mucosectomy performed on the inner cyst wall. The upper part of the cyst wall was excised. The mucosa, submucosa and muscle of the margin of the cyst were approximated with interrupted absorbable sutures. Histopathologic examination was consistent with a gastric duplication cyst containing gastric mucosa (Fig. 4). No ectopic pancreatic tissue was present. The child made an uneventful recovery. Postoperative ultrasonography 7 days later demonstrated a normal stomach lumen with no cystic lesions, and normal passage of fluid via the pylorus into the duodenum. She is currently 10 months old without any sign of gastric obstruction.
Gastric/pyloric duplications are usually visible at laparotomy since they are usually adjacent to the stomach/pylorus [5]. However, in this case, at laparotomy, the duplication was not visible or not identified in the stomach/pylorus from outside. So, intraoperative endoscopy was performed, which revealed the duplication cyst was entirely intraluminal. Our case is an entirely “intraluminal” pyloric duplication.
Gastric duplications are commonly located along the greater curvature of the stomach and 82% are cystic type while have no communication with the stomach with separate mucosal linings even though they may share a common muscular layer and blood supply [5].
We could not find any communication between the pyloric duplication cyst and the biliary system preoperatively and intraoperatively. Duodenal duplication cyst represents 5%-12% of all gastrointestinal tract duplications and often communicates with either the small bowel or the pancreatic duct; rarely with the biliary system [6]. Some gastric duplication cysts are closely connected with the pancreas and its excretory ducts [7]. We used MRCP preoperatively to assess if the cyst communicated with the biliary system or not. However, the image obtained was so poor because of the low specificity of MRCP during the neonatal period, that we decided to perform intraoperative cholangiography instead.
The presence of heterotopic gastric mucosa can cause bleeding or perforation and early resection as soon as possible is indicated to prevent complications, even if the subject is asymptomatic [8910]. In our case, feeding was possible with an ED tube, so we decided to wait until the patient grew back to body weight before contemplating surgical intervention. H2 blockers were also administered to reduce the secretion of gastric acid. Our experience indicates that early intervention is not always necessary even for symptomatic patients if the patient's condition can be stabilized and maintained-in this case by using an ED tube.
The surgical treatment is total excision of the duplication. If total excision is not possible, then mucosectomy is performed in order to prevent recurrence of obstruction or malignant transformation. Shah et al. [11] reported about a neonate with pyloric duplication who had a limited pyloroantrectomy. In their case, it was not possible to dissect the common wall between the duplication and the gastric wall. As a result, the pyloric duplication together with a wedge of gastric antrum was excised, resulting in a limited pyloroantrectomy.
In our case, we performed a transverse gastrotomy, and approached the cyst from inside the stomach. It might have been possible to incise the cyst with endoscopy, but there would have been a risk for recurrence of obstruction due to insufficient length of the incision or gastric wall perforation or for resulting in gastric diverticulum formation for the long time.
Figures and Tables
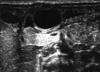
Fig. 1
“Intraluminal” cyst arising from the pyloric orifice seen on ultrasonography. The wall of the lesion had a hypoechoic outer rim and a thick echogenic inner rim or gastrointestinal signature and the lumen was hypoechoic.
References
1. Perek A, Perek S, Kapan M, Göksoy E. Gastric duplication cyst. Dig Surg. 2000; 17:634–636.
2. Mărginean CO, Mărginean C, Horváth E, Gozar L, Gozar HG. Antenatally diagnosed congenital pyloric duplication associated with intraluminal pyloric cyst--rare entity case report and review of the literature. Rom J Morphol Embryol. 2014; 55:983–988.
3. Tang XB, Bai YZ, Wang WL. An intraluminal pyloric duplication cyst in an infant. J Pediatr Surg. 2008; 43:2305–2307.
4. Segal SR, Sherman NH, Rosenberg HK, Kirby CL, Caro PA, Bellah RD, et al. Ultrasonographic features of gastrointestinal duplications. J Ultrasound Med. 1994; 13:863–870.
5. Skandalakis JE, Gray SW. Embryology for surgeons. 2nd ed. Baltimore: Williams & Wilkins;1993.
6. Guarise A, Faccioli N, Ferrari M, Romano L, Parisi A, Falconi M. Duodenal duplication cyst causing severe pancreatitis: imaging findings and pathological correlation. World J Gastroenterol. 2006; 12:1630–1633.
7. Alessandrini P, Derlon S. Gastric duplication communicating with an aberrant pancreas. Eur J Pediatr Surg. 1991; 1:309–311.
8. Brink DA, Balsara ZN. Prenatal ultrasound detection of intra-abdominal pulmonary sequestration with postnatal MRI correlation. Pediatr Radiol. 1991; 21:227.
9. Goyert GL, Blitz D, Gibson P, Seabolt L, Olszewski M, Wright DJ, et al. Prenatal diagnosis of duplication cyst of the pylorus. Prenat Diagn. 1991; 11:483–486.
10. Correia-Pinto J, Tavares ML, Monteiro J, Moura N, Guimarães H, Estevão-Costa J. Prenatal diagnosis of abdominal enteric duplications. Prenat Diagn. 2000; 20:163–167.
11. Shah A, More B, Buick R. Pyloric duplication in a neonate: a rare entity. Pediatr Surg Int. 2005; 21:220–222.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download