Abstract
Infantile hypertrophic pyloric stenosis (IHPS) is one of the common surgical abdomen in infancy, characterized by progressive non-bilious vomiting. The etiology is unknown, but it likely develops after birth. The pylorus of the stomach becomes thick and triggers progressive vomiting. Abdominal ultrasonography (US) is widely used as a diagnostic tool. Currently, there is a rare IHPS patient with severe metabolic derangement because of general use of abdominal US and its accuracy. We experienced a natural course of a 62- day-old male infant with IHPS who was suffering from intermittent vomiting, loss of weight but had not been properly treated for 1 month. It is needed to make an effort to diagnose differentially in recurrent vomiting infant and check-up regularly, and also educate parents properly.
Vomiting is very common in infants. Most of them are periodic or spontaneously resolved. But, sometimes it would be first presentation of serious surgical condition or life-threatening disease. Vomiting presents differential diagnoses based on obstructive or non-obstructive processes. First we consider anatomical obstruction of gastrointestinal tract such as midgut volvulus, intestinal atresia, infantile hypertrophic pyloric stenosis (IHPS), incarcerated inguinal hernia, intussusception should be considered. History of bilious vomiting would be helpful to distinguish the location of the obstruction. Functional causes of vomiting in infant are also considerable, such as gastroesophageal reflux, feeding intolerance, or infection. Otherwise inborn error of metabolism, congenital adrenal hyperplasia, or increased intracranial pressure may be considerable.
It is important to differentiate whether the vomiting is under surgical conditions or not. Red flag signs, such as bile-tinged vomiting, failure to thrive, persistent or projectile vomiting, often suggest serious diseases to require surgical intervention, and may helpful to distinct [1]. IHPS is a common disorder that provokes progressive projectile and non-bilious vomiting. Its etiology is unknown, but surgical intervention is required. Today, parental concern and accurate sonographic evaluation can help early detection of IHPS, before the development of metabolic derangement or olive mass [2].
We experienced a male infant with IHPS who was suffering from severe metabolic derangement without appropriate treatment for 1 month by his parents.
A 62-day-old male infant visited at the emergency room because of lethargy and loss of weight. The baby was born at 38 weeks of gestation with 3.3 kg of weight, and his neonatal screening test, including inborn errors of metabolism, was normal. He had no perinatal problems. He did well for 1 month after birth. But he had vomited milk material 3 times a day for roughly from 1 month ago. At that time, abdominal ultrasonography (US) was checked at an outside clinic, and it didn't show abnormal findings. His parents tried to reduce amount of feeding volume because his abdominal US was normal, then vomiting seemed to improve. Thereafter they fed one-third of the usual volume during 15 days, but vomiting developed again after he wanted to eat more. The vomiting was projectile and non-bilious milk material that was regurgitated shortly after feeding. After vomiting, the baby looked to be continuously hungry and eager to eat. However, his parents reduced his oral intake to relieve the vomiting. Total milk feeding had decreased approximately 200 to 400 mL per day for 2 weeks and the weight was decreased from 3.8 to 3.03 kg for 32 days. Vital signs were followings: blood pressure, 80/50 mmHg; pulse rate, 120 beats/min; respiration rate, 32 breaths/min; and temperature, 36.2℃. He was lethargic and showed severe dried lip with sunken fontanelle. Skin turgor was decreased. Lung sound was clear and heart beat was regular. His abdomen was soft and flat, and we didn't notice a mass like lesion on abdomen. Initial laboratory data were as follows: Na, 124.9 mmol/L (reference range, 134-144); K, 2.4 mmol/L (3.5-6.0); Cl, <60 mmol/L (98-106); BUN, 45 mg/dL (5-18); Cr, 0.7 mg/dL (0.2-0.4); Ca, 12.3 mg/dL (8.8-10.8); total protein, 7.4 g/dL (6.1-7.9); albumin, 4.9 g/dL (3.9-5.0); total bilirubin, 3.23 mg/dL (0-10); direct bilirubin, 1.16 mg/dL (0-0.2); AST, 31 U/L (22-63); and ALT, 16 U/L (10-40). On arterial blood gas anaysis, pH 7.55 (pH 7.35-7.45), CO2 39 mmHg (27-41) and HCO3- 34 mEq/L (22-29) were noted. Urinary analyses were not specific, and urinary sodium was 27.1 mmol/L (40-220); potassium was 23.1 mmol/L (25-120), and chloride was less than 10 mmol/L (20-250).
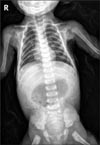
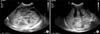
Abdominal X-ray showed marked gastric distension (Fig. 1), and abdominal US revealed hypertrophied pyloric canal with 2.5 cm of length and 0.6 cm of thickness (Fig. 2).
To correct the electrolyte disturbances and severe dehydration, half saline and 5% of dextrose were intravenously administrated. After 36 hours of fluid resuscitation, serum electrolytes and blood acidity were corrected. Forty eight hours after admission, laparoscopic pyloromyotomy was done. After 6 hours, he began to eat and within 24 hours after surgery, he could eat milk enough. He recovered uneventfully and discharged with weight gaining up to 4 kg.
IHPS is one of the most common neonatal surgical abdomen and usually diagnosed between 3 and 5 weeks of age, and very rarely occur after 12 weeks of age [3]. Vomiting after feeding is the most important symptom in a patient with IHPS and it progresses to projectile and recurrent. Recurrent vomiting and dehydration can cause electrolyte imbalance such as hypochloremic alkalosis, a loss in weight and lethargy [4]. In addition to IHPS, hypokalemic hypochloremic metabolic alkalosis with decreased urinary chloride excretion is caused by various conditions such as inadequate diet, cystic fibrosis, and congenital chloridorrhea [567].
Recently, the patient with IHPS has shown fewer clinical hallmarks of the disease because of parental concern and the high sensitivity of abdominal US [28]. Hyponatremia in our patient was caused by inadequate consumption of milk. The parents fed him only 200 mL per day for the last week. Commercial cow milk based formula typically has 480 mg of sodium per liter; the patient seemed to be taken only 1/4 to 1/5 of his daily sodium requirement.
Rapid fluid resuscitation is warranted in infants with hypoelectrolytemia in emergent medical situation, because hyponatremia can cause severe complications of central pontine myelinolysis.
In our patient, abdominal US had been checked when he was 1 month old, but it didn't revealed the evidence of IHPS. We estimated that the pyloric thickness and length might be less than the criteria for diagnosis of IHPS at 1 month of age, and the doctor might considered that vomiting was provoked by gastroesophageal reflux disease. In addition, outside clinician educated the patient to visit again when vomiting continued or weight increased improperly but parents didn't recognize it and thought to be improved.
The normal gastric pylorus is less than 2 mm of the transverse thickness [9]. But IHPS is progressive disease. Thus, even though pyloric thickness or length on abdominal US does not fulfill the criteria of IHPS, doctor may consider the possibility of IHPS based on the patient's age and size [10]. If pyloric thickness is more than 2 mm but less than 3 mm, a repeat check-up might be warranted.
In 2% to 5% of IHPS cases, unconjugated hyperbilirubinemia may present, but it is generally known to improve after surgery and feeding [1112]. We also experienced this phenomenon in the patient and the jaundice improved via fluid resuscitation before surgical correction. Hyperbilirubinemia in IHPS may be associated with defective hepatic glucuronyl transferase activity and may suggest an early manifestation of Gilbert syndrome in this patient [8].
IHPS is one of the most common disorders in vomiting infants, but insufficient parental education can trigger serious emergent conditions, such as those the patient presented with. The rate of gaining weight is one of the most important factors in a vomiting infant. An infant with vomiting that poor gain of weight should be serially checked up, because IHPS is a progressive disorder, can be presented older than 2 months, and has fewer clinical hallmarks. The importance of appropriate education about feeding babies and serial follow-up cannot be overstated with a vomiting baby.
Figures and Tables
References
1. Whinney J, Paul SP, Candy DC. Clinical update: vomiting in infants. Community Pract. 2013; 86:44–47.
2. Hernanz-Schulman M, Sells LL, Ambrosino MM, Heller RM, Stein SM, Neblett WW 3rd. Hypertrophic pyloric stenosis in the infant without a palpable olive: accuracy of sonographic diagnosis. Radiology. 1994; 193:771–776.
3. Son SK, Park JH, Choi BH, Choi KH, Lee KH. A clinical analysis of neonatal surgical gastrointestinal diseases in Daegu/Busan area. Korean J Pediatr Gastroenterol Nutr. 2004; 7:179–185.
4. Choi KJ. Infantile hypertrophic pyloric stenosis. Ewha Med J. 2012; 35:16–20.
5. Park ES, Seo JH, Lim JY, Park CH, Woo HO, Youn HS. Hypoelectroly temia due to inadequate diet. Pediatr Nephrol. 2006; 21:430–432.
6. Fustik S, Pop-Jordanova N, Slaveska N, Koceva S, Efremov G. Metabolic alkalosis with hypoelectrolytemia in infants with cystic fibrosis. Pediatr Int. 2002; 44:289–292.
7. Mäkelä S, Kere J, Holmberg C, Höglund P. SLC26A3 mutations in congenital chloride diarrhea. Hum Mutat. 2002; 20:425–438.
8. Kim YH, Jung MS, Byun SO. A clinical study of hypertrophic pyloric stenosis. J Korean Pediatr Soc. 2002; 45:1389–1396.
9. Maclennan AC. Investigation in vomiting children. Semin Pediatr Surg. 2003; 12:220–228.
10. Iqbal CW, Rivard DC, Mortellaro VE, Sharp SW, St Peter SD. Evaluation of ultrasonographic parameters in the diagnosis of pyloric stenosis relative to patient age and size. J Pediatr Surg. 2012; 47:1542–1547.
11. Klaudia AH, Chris AL. Hypertrophic pyloric stenosis. In : Kliegman R, Nelson WE, St. Geme JW, editors. Nelson textbook of pediatrics. 19th ed. Philadelphia: Saunders;2011. p. 1274–1275.
12. Hua L, Shi D, Bishop PR, Gosche J, May WL, Nowicki MJ. The role of UGT1A1*28 mutation in jaundiced infants with hypertrophic pyloric stenosis. Pediatr Res. 2005; 58:881–884.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download