Abstract
Pacinian corpuscle-like structures were identified in the digital tendon sheaths and nail beds of hands obtained from eight of 12 human fetuses of gestational age 20–34 weeks (crown-rump length, 150–290 mm). The aberrant corpuscles were present in tight fibrous tissue connecting the flexor tendon sheath to the dorsal aponeurosis (138 corpuscles in the thumbs and all fingers of eight fetuses); loose fibrous tissue inside the sheath on the dorsal side of the tendon (37 corpuscles in the thumbs and all fingers of four fetuses); and the nail bed (10 clusters in the thumbs and second fingers of four smaller fetuses). The aberrant corpuscles in the tendon sheath were classified into two types: thin and short, with tightly packed lamellae, of diameter 20–40 µm and length 20–200 µm; and thick and long, with loosely packed lamellae, of diameter 70–150 µm and length 0.5–1.5 mm. The small corpuscles tended to form clusters, each containing 5–10 structures. Their similarity indicated that the tight and loose lamellae in these two types of corpuscles corresponded to typical immature and mature corpuscles, respectively, usually distributed along the palmar digital nerve. However, mature, large corpuscles were absent from the nail bed, and most aberrant corpuscles were smaller than typical corpuscles along the nerve. The aberrant corpuscles were apparently incorporated into the tendon sheath or nail bed during fetal vascular development, but they appeared to degenerate after birth due to mechanical stress from the tendon or nail.
Three stages in the development of digital Pacinian corpuscles in the human fetus have been described: (1) a primordial stage, consisting of one type of cell distributed along “stag-horn-like” nerve twigs and observed in fetuses of crown-rump length (CRL) 70–90 mm; (2) an avascular stage, characterized by lamellae or an outer bulb of perineural origin and observed in fetuses of CRL ≥120 mm; and (3) a vascular stage, characterized by the gradual incorporation of surrounding blood vessels into the lamellae and observed in fetuses of CRL ≥200 mm [1]. To verify these observations, we studied serial transverse sections of the distal and middle segments of the thumb and all fingers obtained from 12 hands of 12 late-stage fetuses of gestational age 20–34 weeks (CRL, 150–290 mm). We found that the corpuscles in late-stage fetuses can be classified into two types: (1) thin, small, immature corpuscles with tightly packed lamellae, of thickness 30–70 µm and length 0.3–0.7 mm; and (2) thick, long, mature corpuscles with loosely packed lamellae, of thickness 80–150 µm and length 0.5–2.0 mm and morphology similar to that in adults [2]. Since both types receive vascular supply, however, all corpuscles in late-stage fetuses apparently attain the aforementioned vascular stage [1].
Digital Pacinian corpuscles are distributed along the palmar digital nerve trunk [34]. However, examination of transverse sections from 12 fetal hands resulted in the incidental detection of aberrant corpuscles or corpuscle-like structures in the flexor tendon sheath of the fingers and thumb, as well as in the nail bed. To our knowledge, Pacinian corpuscle-like structures have never been reported in and along the digital flexor tendon, although this tendon carries nerve terminals and various mechanoreceptors [56789]. Little is known about mechanoreceptors in the nail bed. Therefore, the aim of the present study was to describe the detailed morphology of these aberrant corpuscles.
This study was performed in accordance with the provisions of the Declaration of Helsinki 1995 (as revised in 2013). Transverse sections of 12 hands were obtained from 12 human fetuses (eight males and four females) of gestational age 20–34 weeks (CRL 150–290 mm) (Table 1). The finger joints in all specimens were not severely flexed but were almost extended. These fetuses were part of a collection donated to the Department of Anatomy, Akita University, Akita, Japan, by their respective families during the period 1975–1985, preserved in 10% w/w neutral formalin solution for more than 30 years, and maintained in the department. The available data were limited to the date of donation and the number of gestational weeks. There were no related documents providing the family name, the name of the attending obstetrician or hospital, or the reason for abortion. The use of these specimens for research was approved by the Ethics Committee of Akita University (No. 1378).
Upon retrieval, the hand specimens were incubated at room temperature in Plank-Rychlo solution (7.0% [w/v] AlCl2/6H2O, 3.6% [w/v] HCl, 4.6% [w/v] HCOOH) for 1–2 weeks. To reduce sectioning time, three to five fingers, depending on the size of the fetus, were bundled and embedded en bloc in paraffin. Thus, every section contained 3–5 fingers. Because the thumb was thick, three thumbs of three fetuses were embedded together in paraffin. Samples were serially sectioned, at a thickness of 5 µm, from the tip of the finger to the proximal interphalangeal joint or to the interphalangeal joint in the thumb. Therefore, these sections covered almost the entire distal and middle phalangeal segment of the fingers, or the distal segment and almost half of the proximal segment of the thumb. Three to four sections were mounted on each glass slide. Overall, for each hand, 800–1,500 slides containing 3,000–4,500 sections were prepared. All of the sections were stained with hematoxylin and eosin. Photographs were taken with an ×20 objective lens, with distances measured manually using calipers.
Typical corpuscles were observed along the palmar digital nerve trunk (Fig. 1). These were classified into two types: (1) thin and small corpuscles with tightly packed lamellae, of thickness 30–70 µm and length 0.3–0.7 mm (Fig. 1C); and (2) thick and long corpuscles with loosely packed lamellae, of thickness 80–150 µm and length 0.5–2.0 mm (Fig. 1A). The small (or immature) and large (or mature) types appeared to be mixed and contained within a single or multiple capillaries. The proportion of the large and mature type of corpuscles varied among the fetuses. The numbers of typical corpuscles ranged from 2–10 per section in the distal part of the distal phalangeal segment, and from 0–5 per section near the distal interphalangeal joint. More corpuscles were observed in the thumb and fifth finger than in the second through fourth fingers.
Aberrant corpuscles were present in (1) tight fibrous tissue connecting the flexor tendon sheath to the dorsal aponeurosis (138 corpuscles in the thumbs and all fingers of eight fetuses) (Figs. 1, 2); and (2) loose fibrous tissue inside the sheath or along the phalangeal bone on the dorsal side of the tendon (37 corpuscles in the thumbs and all fingers of four fetuses (Figs. 3, 4). These corpuscles were either solitary or formed clusters. All aberrant corpuscles embedded in tight fibrous tissue were small with tightly packed lamellae (Figs. 1B, D, 2C, E). Although many aberrant corpuscles within the loose tissue were small (Figs. 3B, E, 4B, D, F), some were large with loosely packed lamellae (Fig. 3D). The small aberrant corpuscles were 20–40 µm in diameter and 20–200 µm in length, whereas the large corpuscles were 70–150 µm in diameter and 0.5–1.5 mm in length. Most of the aberrant corpuscles in the tendon sheath of each section were smaller and shorter than typical corpuscles distributed along the palmar digital nerve. Some small corpuscles located in the tight fibrous tissue of the tendon sheath connected to the dorsal aponeurosis formed clusters (Fig. 2E). The central axon or an eosinophilic core was usually evident, even in small aberrant corpuscles (Figs. 1B, 2B, 3E, 4B, D, E).
The largest number of large corpuscles with loosely packed lamellae along the palmar digital nerve was observed in a specimen of gestational age 23 weeks (CRL, 175 mm) (Figs. 3, 6). Moreover, this specimen also contained the largest number of aberrant corpuscles in the tendon sheath. Examination of the middle phalangeal segment near the proximal interphalangeal joint showed thinning of the tendon sheath at the base attached to the phalangeal bone, with the sheath interrupted by loose tissue (Fig. 5). In three fetuses, typical corpuscles along the palmar digital nerve were found to extend into the loose tissue and to enter the ventral end of the dorsal aponeurosis (Fig. 5B, C). Aberrant corpuscles were observed in the tendon sheaths of both smaller and larger specimens (Table 1), with the loose tissue at the base of the tendon sheath receiving capillaries distributed toward the tendon.
Small corpuscles were observed in the nail beds of four smaller fetuses (Table 1). These corpuscles were solitary or formed clusters containing 5–10 corpuscles, with these four fetuses containing a total of 10 clusters. Corpuscles were absent from the nail beds of the third through fifth fingers. Moreover, large or mature corpuscles with loosely packed lamellae were absent from the nail bed. Aberrant corpuscles were associated with thin nerve twigs (Fig. 6E) but were located far distally from the site at which a terminal branch of the dorsal digital nerve entered the nail bed. Normal corpuscles in the thumb were associated with both the palmar and dorsal digital nerve trunks. The nail bed contained an abundant number of capillaries, but there were no or few corpuscles in the tips of fingers distal to the nail bed.
This study showed the presence of aberrant Pacinian corpuscles or corpuscle-like structures in the flexor tendon sheaths and nail beds of fetal hands. Their lamellar structures were very similar to those in typical corpuscles distributed along the palmar digital nerve. The small, immature corpuscles with tightly packed lamellae observed in these fetuses were mixed with large, mature corpuscles with loosely packed lamellae. Specimens with larger numbers of large corpuscles with loosely packed lamellae along the nerve also bore similar large corpuscles along the tendon sheath. These findings suggest that these corpuscle-like structures developed through a process similar to that of typical Pacinian corpuscles. Therefore, rather than being called corpuscle-like structures, the term “aberrant corpuscles” seemed more appropriate.
Tight fibrous tissue contained only small, immature corpuscles. Only the smaller fetuses of earlier gestational age contained aberrant corpuscles in the nail bed, especially that of the thumb. Therefore, our findings suggest that aberrant corpuscles are likely incorporated incidentally into the flexor tendon sheath or nail bed at an early developmental stage but may degenerate after birth due to mechanical stress from the tendon or nail. This possible corpuscle invasion may be associated with the entry of capillaries into the tendon sheath or nail bed during early vascular development. Unfortunately, the present specimens were too large or of too late stage to allow verification.
The term “glomus” is used to indicate an arteriovenous anastomosis, found usually in subcutaneous tissue, especially the nail bed [10]. Glomus cells contain abundant myofibrils, and are thought to originate from pericytes of the vessels involved [1112]. Digital glomus tumors have been associated with pathological Pacinian corpuscles [131415]. However, there is an apparent logical gap in explaining interactions between glomus cells of vascular origin and corpuscles of nerve origin. We recently hypothesized that tyrosine hydroxylase-positive sympathetic nerve fibers, which are rich in the human adult coccygeal body, induce the transformation of arterial smooth muscle cells into glomus cells [16]. Similarly, a remnant of fetal corpuscles in the nail bed, which would be of nerve origin, may induce the tumorous transformation of the nail capillary mesh. Moreover, various types of glomus tumor may originate from these aberrant corpuscles. After birth, however, all aberrant corpuscles in the tendon sheath likely disappear due to marked mechanical stress from the digital flexor tendons. Overall, some fetal Pacinian corpuscles likely degenerate, whereas others are transformed to tumor cells. Further studies, especially those using immunohistochemical methods, are necessary to characterize these specific corpuscles.
Figures and Tables
Fig. 1
Pacinian corpuscle-like structures in tight fibrous tissue of the digital tendon sheaths of fetuses of gestational ages 34 weeks (crown-rump length [CRL], 290 mm) (A, B) and 30 weeks (CRL, 250 mm) (C, D). Panels (B) and (D) are higher magnification views of squares in panels (A) and (C), respectively. In panels (A) and (C), the palmar digital nerve (PDN) trunk is accompanied by usual Pacinian corpuscles (arrowheads). In tight fibrous tissue connecting the palmar tendon sheath around the flexor tendon (tendon) and the dorsal aponeurosis (DAP), the corpuscle-like structures (arrows) are cut longitudinally (B, upper and D) or transversely (B, lower). art, artery; middle 2 (or 4) in a circle, middle phalangeal bone of the second (or fourth) finger; DDN, dorsal digital nerve trunk. Transverse sections. Hematoxylin and eosin staining. Scale bars=1 mm (A, C), 0.1 mm (B, D).
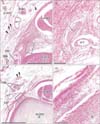
Fig. 2
Pacinian corpuscle-like structures in a tight fibrous tissue of the digital tendon sheath of a fetus of gestational age 28 weeks (crown-rump length, 230 mm). (A) The topographical anatomy, including the palmar sheath for a flexor tendon (tendon) in the distal phalangeal segment of the thumb (distal 1 in a circle). (D) The tendon sheath in the distal phalangeal segment of the second finger (distal 2 in a circle), as well as the palmar digital nerve (PDN) trunk accompanied by usual Pacinian corpuscles (arrowhead). Panels (B) and (C) are higher magnification views of the squares in panel (A), whereas panel (E) is a higher magnification of a square in panel (D). In panel (D), the structures in a circle are seen on the dorsal side of the square. In tight fibrous tissue connecting the palmar tendon sheath and the dorsal aponeurosis (DAP), the corpuscle-like structures are cut transversely (arrows in panels B, C, and E) or longitudinally (arrowhead in panel C). art, artery. Transverse sections. Hematoxylin and eosin staining. Scale bars=1 mm (A, D), 0.1 mm (B, C, E).
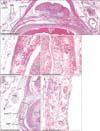
Fig. 3
Pacinian corpuscle-like structures inside the digital tendon sheath of a fetus of gestational age 23 weeks (crown-rump length, 175 mm). (A, C) The topographical anatomy around the palmar sheath of a flexor tendon (tendon) in the middle phalangeal segment of the fifth finger (middle 5 in a circle). Panel (A) is 0.1 mm distal to panel (C). The palmar digital nerve (PDN) trunk is accompanied by usual Pacinian corpuscles (arrowheads in C). Panel (B) is a higher magnification view of a square in panel (A), whereas panels (D) and (E) are higher magnification views of squares in panel (C). In loose tissue between the tendon and middle phalangeal bone, the corpuscle-like structures are scattered (arrows in panel B) or extend along the left-right axis near the bone surface (stars in panels C and D). In fibrous tissue at the base of the tendon sheath (C, E), the corpuscle-like structures (arrows) are cut longitudinally or transversely (arrows in panel E). Transverse sections. Hematoxylin and eosin staining. Scale bars=1 mm (A, C), 0.1 mm (B, D, E).
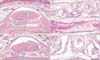
Fig. 4
Pacinian corpuscle-like structures inside the digital tendon sheaths of fetuses of gestational ages 29 weeks (crown-rump length [CRL], 240 mm) (A, B), 30 weeks (CRL, 250 mm) (C and D, identical to the specimen in Fig. 1C, D) and 28 weeks (CRL, 230 mm) (E and F; identical to the specimen in Fig. 2). Panels (B), (D), and (F) are higher magnification views of squares in panels (A), (C), and (E), respectively. Loose tissue inside the palmar sheath of flexor tendons (tendon) contains corpusclelike structures (arrows in panels B, D, and F). art, artery; middle 2, 3, or 4 in a circle, middle phalangeal bone of the second, third or fourth finger; DAP, dorsal aponeurosis; DDN, dorsal digital nerve trunk; PDN, palmar digital nerve trunk. Transverse sections. Hematoxylin and eosin staining. Scale bars=1 mm (A, C, E), 0.1 mm (B, D, F).
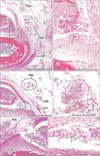
Fig. 5
Pacinian corpuscle-like structures in fibrous tissue connected to the dorsal aponeurosis of a fetus of gestational age 28 weeks (crown-rump length, 230 mm; identical to the specimen in Figs. 2 and 4E, F). Panels (A–C) show the topographical anatomy, including a flexor tendon (tendon) in the middle phalangeal segment of the fourth finger (middle 4 in a circle). Panels (A) and (C) represent the most distal and proximal sites, respectively, in the figure. Intervals between panels were 1.0 mm (A–B) and 0.2 mm (B–C). The flexor tendon sheath is evident (A) but is interrupted by loose tissue (B, C). The palmar digital nerve trunk (PDN) is accompanied by usual Pacinian corpuscles (arrowheads in panels A–C). Panels (D), (E), and (F) are higher magnification views of squares in panel (A), (B), and (C), respectively. (B) The structures in circles are seen outside the square. The long axis in panel (D) corresponds to the left-right axis in panel (A) and shows usual corpuscles (arrows), one cut longitudinally. Multiple corpuscle-like structures (arrows in panels E and F), enclosed by a square and circles in panels (B) and (C), are embedded in fibrous tissue connected to the dorsal aponeurosis. art, artery; DDN, dorsal digital nerve trunk; DAP, dorsal aponeurosis. Transverse sections. Hematoxylin and eosin staining. Scale bars=1 mm (A–C), 0.1 mm (D–F).
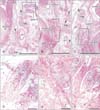
Fig. 6
Pacinian corpuscle-like structures in a nail bed of fetuses of gestational ages 23 weeks (crown rump length [CRL], 175 mm; panels A–C, identical to the specimen in Fig. 3) and 29 weeks (CRL 240 mm; panels D and E, identical to the specimen in Fig. 4A, B). Panels (B) and (C) are higher magnification views of a square in panel (A), and panel (E) is a higher magnification view of a square in panel (D). Panel (A) shows the topographical anatomy including a nail bed. The palmar side of the distal phalangeal segment (distal 1 in a circle) contains multiple corpuscles (arrowheads; higher magnification in panel C). Panel (D) shows the dorsal half of the distal phalangeal segment (distal 2 in a circle). Panels (B) and (E) show higher magnification views of corpuscle-like structures (arrows) in the nail bed (nail). Transverse sections. Hematoxylin and eosin staining. Scale bars=1 mm (A, D), 0.1 mm (B, C, E).
Table 1
Observation of corpuscle-like structures in 12 fetal hands

D1–D5, distal segment of the thumb and the second-fifth fingers; M1–M5, middle segment of the thumb and the second-fifth fingers; M+, another corpuscle-like structure was present in a loose tissue inside of the sheath in the middle phalangeal segment. a)Corpuscle-like structures in tight fibrous tissue of the flexor tendon sheath to connect with the dorsal aponeurosis. b)Corpuscle-like structures in the nail bed.
Acknowledgements
This study was supported in part by a Grant-in-Aid for Scientific Research (JSPS KAKENHI No. 16K08435) from the Ministry of Education, Culture, Sports, Science and Technology in Japan.
References
1. Cauna N, Mannan G. Development and postnatal changes of digital Pacinian corpuscles (corpuscula lamellosa) in the human hand. J Anat. 1959; 93:271–286.
2. Kobayashi K, Cho KW, Yamamoto M, Mitomo K, Murakami G, Abe H, Abe S. Tree of Vater-Pacinian corpuscles in the human finger and thumb: a comparison between specimens from late-stage fetuses and elderly cadavers. Surg Radiol Anat. 2017; Forthcoming.
3. Hirasawa Y, Sakakida K, Tokioka T, Ohta Y. An investigation of the digital nerves of the thumb. Clin Orthop Relat Res. 1985; (198):191–196.
4. Stark B, Carlstedt T, Hallin RG, Risling M. Distribution of human Pacinian corpuscles in the hand. A cadaver study. J Hand Surg Br. 1998; 23:370–372.
5. Bistevins R, Awad EA. Structure and ultrastructure of mechanoreceptors at the human musculotendinous junction. Arch Phys Med Rehabil. 1981; 62:74–83.
6. Zimny ML, DePaolo C, Dabezies E. Mechano-receptors in the flexor tendons of the hand. J Hand Surg Br. 1989; 14:229–231.
7. Babu KS, Devanandan MS. Sensory receptors situated in the interphalangeal joint capsule of the fingers of the bonnet monkey (Macaca radiata). Acta Anat (Basel). 1995; 153:49–56.
8. Saxod R. Ontogeny of the cutaneous sensory organs. Microsc Res Tech. 1996; 34:313–333.
9. Provitera V, Nolano M, Pagano A, Caporaso G, Stancanelli A, Santoro L. Myelinated nerve endings in human skin. Muscle Nerve. 2007; 35:767–775.
10. Williams PL. Gray's anatomy. 38th ed. London: Churchill Livingstone;1995. p. 1468. p. 1558.
11. Ho KL, Pak MS. Glomus tumor of the coccygeal region: case report. J Bone Joint Surg Am. 1980; 62:141–142.
12. Bell RS, Goodman SB, Fornasier VL. Coccygeal glomus tumors: a case of mistaken identity? J Bone Joint Surg Am. 1982; 64:595–597.
13. Barbolini G, Tischendorf F, Curri SB. Histology, histochemistry, and function of the human digital arteriovenous anastomoses (Hoyer-Grosser's organs, Masson's glomera). I. The possible relationship with Pacinian corpuscles. Microvasc Res. 1971; 3:142–153.
14. Greider JL Jr, Flatt AE. Glomus tumor associated with pacinian hyperplasia: case report. J Hand Surg Am. 1982; 7:113–117.
15. Komforti M, Cummings TJ. An extraordinary association of glomus tumor and Pacinian hyperplasia in the hand of a female patient. Am J Dermatopathol. 2015; 37:719–720.
16. Jin ZW, Cho KH, Jang HS, Murakami G, Yamamoto M, Abe S. Coccygeal body revisited: an immunohistochemical study using donated elderly cadavers. Anat Rec. 2017; Forthcoming.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download