Abstract
Busulfan is an anticancer drug, which causes the apoptosis germ cells and azoospermia in humans and animals. Abnormal morphology of spermatozoa related to the male infertility. The sperm morphology is evaluation of sperm size, shape and appearance characteristics should be assessed by carefully observing a stained sperm sample under the microscope. Evaluation of sperm morphology has been considered as one of the most important factors for a successful fertilization and determining sperm quality. The mice were assigned to tow experimental groups: control and busulfan. Each group included six mice that were housed under standard conditions. The volume was estimated using the nucleator method. The sperm's flegellum and mid-piece length was estimated by counting the number of intersections between the tails and Merz grid test line in an unbiased counting frame, superimposed on live images of sperms. Our results demonstrated a significant different in the volume and surface of the sperm's head and the length of the sperm's flagellum in the control and busulfan groups. Busulfan can effect on the volume of the sperm's head and the length of the sperm's flagellum in rat.
The dysfunction of reproductive system and infertility are the most important adverse effects of chemotherapy [1]. Busulfan (1,4-butanediol dimethanesulfonate) is a bifunctional alkylating agent, that use to treat chronic myeloid leukemia and before a bone marrow or stem cell transplant, which inhibits cell division by sticking to one of the DNA strands. Therefore, the tissues such as bladder, liver, skin, nervous system, and gonadal and cells with high division activities like as testes and germ cells and somatic cells are influenced by adverse effects of busulfan. In addition, treatment by busulfan affects fertility in the male and makes prolonged and sometimes irreversible depression of sperm counts in humans [23]. It has been show that the single doses of busulfan can preferentially kills spermatogonial stem cell and cause sterilize mice by deplete germ cells and disrupt the junctions between Sertoli cells, and cause long-term morphological damage to sperm produced by surviving spermatogonia [1]. Effects of busulfan on the spermatogenesis have been evaluated on testis and male reproductive system [345], but the effects of busulfan on sperm structure are still unclear. However, more information about the effect of chemotherapeutic agent on sperm morphology and structure is needed to treat these infertile men [5678].
Abnormal morphology of spermatozoa can be related to the male infertility. In the last years, there has been much debate on a new potential parameter and methods of sperm morphology analysis and relation of these parameters by male fertility. The human sperms dimension and their potential roles in male infertility are not still fully understood [1]. Therefore, evaluation of sperm morphology can be considered as one of the most important factors for a successful fertilization and determining sperm quality [9101112]. Although the World Health Organization (WHO) recommendations to evaluation various other constituent part of sperm morphology but little attention has been paid to the parameters of the volume of sperm's head and length of sperm's mid-piece and sperm's tail, in spite of they play a part in to sperm motility [1314]. Thus, studies can have been constrained to special areas; including sperm tail and sperm head [1516]. Therefore, measurement of the volume of sperm's head and length of sperm's mid-piece and sperm's flagellum can be useful for to known sperm quality. Stereological methods considered in this study because unbiased stereological techniques were used in this study are easy, fast and accurate [11]. In the light of the above-mentioned reports, this investigation was designed to evaluate the effects of busulfan on the volume of sperm's head and length of the sperm's tail and mid-piece by using stereological methods.
In this study, 12 adult mice (28–30 g) were obtained from the laboratory animal center of Shahid Beheshti University of Medical Sciences, Tehran, Iran. The Ethics Committee of the University approved the animal experiment (IR.SBMU.SM.REC.1395.584). The mice were assigned to tow experimental groups: (1) control and (2) busulfan. Each group included six mice that were housed under standard conditions, room temperature (22℃–24℃), and a 12:12 hour light-dark schedule and had free access to water and food.
Oligospermia can be induced in mice by single dose (20 mg/kg) intraperitoneal injection of the busulfan. All mice were kept for 8 weeks after the busulfan injection then sperm morphology examination was done.
The volume was estimated using the nucleator method. The sperm's head was cut into isotropic uniform random pieces using the orientator method. They were stained with Diff-Quik. The sperm's head were sampled using an optical dissector. For each sampled sperm's head, two horizontal directions (intercept, ln) were considered from the central point within the sperm's head to the sperm's head borders. From a series of these measurements (120–200 intercepts in each group), the mean sperm's head volume in the number weighted distribution was estimated [1819] (Fig. 2):
Each slide was studied with a video-microscopy system made up of a microscope (E-200, Nikon, Tokyo, Japan) linked to a video camera. An oil immersion objective lens ×100 was used to achieve a better recognition of the head, mid-piece and flagella. The method of estimation of the length of sperm's mid-piece and flagella on microscopic slides is an example of the application of length estimation in 2D space. The whole slide was covered by sampling it in a systematic random manner. Briefly, the microscopic fields were sampled in a systematic random manner, by moving the microscope stage in an equal interval along the X- and Y-direction of the stage, using a stage micrometer. One hundred sperms were sampled on each slide (each subject). There are some guidelines that will suffice for stereological sample size to have an acceptable precision. According to these rules 100–200 probe interaction (e.g., sampling of 100 intersections of Merz grid with sperm's mid-piece and flagella) is sufficient. This procedure was continued until the whole slide was studied. In each sample field a test grid composed of two elements was superposed on the image on the monitor. If a sperm's head lay inside the frame and did not touch the forbidden lines (left and inferior borders of the frame), it was sampled. The mean sperm's mid-piece and flagella length was estimated using the following formulae [1819]:
where a/l is the Merz grid constant which was obtained as follows: the area of each basic tile of the grid was X multiplied by Y. Within this tile, there were two semicircles of length of π.d (perimeter of a circle), where d was the diameter of the semicircle. Thus the Merz grid constant a/l was (X_Y) π.d. asf was the area of the basic tile divided by the area of the counting frame. ΣI was the summation of the intersection of the tails with the semicircles. ΣN was the total number of the counted sperms in the unbiased counting frame (Fig. 2).
The results showed that the sperm heads volume was significantly different between control and busulfan groups (P<0.02) (Table 1). Therefore, sperm heads volume was associated with abnormal sperm morphology.
The present study investigated the structural changes of the spermatozoa in the busulfan-induced oligospermia in rats using stereological methods. Our results showed a significant different in the volume and surface of the sperm's head in the control and busulfan groups. Our results also demonstrated that a significant different in the length of the sperm's flagellum in the control and busulfan groups. These changes in the sperm structural have been shown to be accompanied and related by impairments in the chance of sperm fertility [1]. Busulfan is a chemotherapeutic agent which has many side effects on reproductive system [2]. Busulfan is a potent agent, which causes the reduction and apoptosis of spermatogonial stem cells populations [2]. In the previous study showed that the morphological change of spermatozoa following administration of busulfan in rat [2]. Eight weeks after the intraperitoneal injection of busulfan, we found the structural changes in rat's spermatozoa. However, busulfan causes change and damage to the sperm structure when administered by an intraperitoneal injection [20]. Our results were in accordance with the previous studies reporting the adverse effects of busulfan [220] and showed that busulfan injections in male rat could induce azoospermia and structural changes in spermatozoa.
Moreover, there are several approaches for malignancy therapy such as chemotherapy and radiotherapy. It has been shown that fractionated irradiation results in depletion of endogenous spermatogenesis similar to using busulfan injection. There is little information about the mechanism of busulfan toxicity on the sperm structure, but its toxic effect on the reproductive system may associate with the fertility outcomes. Our result showed the relationship between busulfan toxicity and structural disorders in spermatozoa.
Busulfan can effect on the sperm structure such as volume of the sperm's head and the length of the sperm's flagellum. The reason why spermatozoa had abnormality in volume of the sperm's head and the length of the sperm's flagellum is unknown and need to more evaluation the mechanism of effect of busulfan on sperm structure. However, stereological methods can provide valuable quantitative information about the morphological changes in the sperm structure that cannot be obtained using sperm analysis by a time consuming other techniques like as image analyzers or tracing method on the electron or light microscopic samples. The stereological procedure not only faster than conventional methods but also no need special software to create these grids.
In conclusion, the use of stereological methods is recommended for the morphological change evaluation of the sperm by light microscopy. This method can to be a useful and efficient way to estimate the volume of sperm's head and sperm's mid-piece and tail lengths in rats.
Figures and Tables
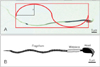
Fig. 1
Microphotograph illustrating morphology of sperm in control (A) and busulfan (B) group in mice (Diff-Quik staining, ×40).

Fig. 2
Estimation of the sperms mid-piece and flagellum length. (A) Four cycloids were located at a rectangle. The length of each cycloid was equal to twice the length of its minor axis (r). The area associated with the cycloids was calculated by multiplying the X by Y and divided by the length of the four cycloids to achieve the area per length. The total number of the intersections between the sperms mid-piece and flagellum axes and the cycloid were counted. The cycloid was positioned parallel to the vertical axis (×100). (B) Schematic representation of the structure of a spermatozoon (×100).
Acknowledgements
This article has been extracted from the thesis by Mrs Sakineh Panahi in School of Medicine Shahid Beheshti University of Medical Sciences (registration No. 1395.115).
References
1. Howell SJ, Shalet SM. Spermatogenesis after cancer treatment: damage and recovery. J Natl Cancer Inst Monogr. 2005; (34):12–17.
2. Bucci LR, Meistrich ML. Effects of busulfan on murine spermatogenesis: cytotoxicity, sterility, sperm abnormalities, and dominant lethal mutations. Mutat Res. 1987; 176:259–268.
3. Iwamoto T, Hiraku Y, Oikawa S, Mizutani H, Kojima M, Kawanishi S. DNA intrastrand cross-link at the 5′-GA-3′ sequence formed by busulfan and its role in the cytotoxic effect. Cancer Sci. 2004; 95:454–458.
4. Choi YJ, Ok DW, Kwon DN, Chung JI, Kim HC, Yeo SM, Kim T, Seo HG, Kim JH. Murine male germ cell apoptosis induced by busulfan treatment correlates with loss of c-kit-expression in a Fas/FasL- and p53-independent manner. FEBS Lett. 2004; 575:41–51.
5. Petersen PM, Skakkebaek NE, Giwercman A. Gonadal function in men with testicular cancer: biological and clinical aspects. APMIS. 1998; 106:24–34.
6. Schrader M, Müller M, Straub B, Miller K. The impact of chemotherapy on male fertility: a survey of the biologic basis and clinical aspects. Reprod Toxicol. 2001; 15:611–617.
7. Chemes HE, Alvarez Sedo C. Tales of the tail and sperm head aches: changing concepts on the prognostic significance of sperm pathologies affecting the head, neck and tail. Asian J Androl. 2012; 14:14–23.
8. Mosher WD. Fecundity and infertility in the United States. Am J Public Health. 1988; 78:181–182.
9. Martí JI, Aparicio MI, Leal CL, García-Herreros M. Seasonal dynamics of sperm morphometric subpopulations and its association with sperm quality parameters in ram ejaculates. Theriogenology. 2012; 78:528–541.
10. Rønn LC, Ralets I, Hartz BP, Bech M, Berezin A, Berezin V, Møller A, Bock E. A simple procedure for quantification of neurite outgrowth based on stereological principles. J Neurosci Methods. 2000; 100:25–32.
11. Howard CV, Reed M. Unbiased stereology: three-dimensional measurement in microscopy. 2nd ed. Oxford: Bios Scientific Publisher;2005. p. 183–190.
12. Kuster CE, Singer RS, Althouse GC. Determining sample size for the morphological assessment of sperm. Theriogenology. 2004; 61:691–703.
13. Mossman JA, Pearson JT, Moore HD, Pacey AA. Variation in mean human sperm length is linked with semen characteristics. Hum Reprod. 2013; 28:22–32.
14. de Paz P, Mata-Campuzano M, Tizado EJ, Alvarez M, Alvarez-Rodríguez M, Herraez P, Anel L. The relationship between ram sperm head morphometry and fertility depends on the procedures of acquisition and analysis used. Theriogenology. 2011; 76:1313–1325.
15. Maroto-Morales A, Ramón M, García-Alvarez O, Soler AJ, Esteso MC, Martínez-Pastor F, Pérez-Guzmán MD, Garde JJ. Characterization of ram (Ovis aries) sperm head morphometry using the Sperm-Class Analyzer. Theriogenology. 2010; 73:437–448.
16. Noorafshan A, Karbalay-Doust S. A simple method for unbiased estimating of ejaculated sperm tail length in subjects with normal and abnormal sperm motility. Micron. 2010; 41:96–99.
17. Seed J, Chapin RE, Clegg ED, Dostal LA, Foote RH, Hurtt ME, Klinefelter GR, Makris SL, Perreault SD, Schrader S, Seyler D, Sprando R, Treinen KA, Veeramachaneni DN, Wise LD. Methods for assessing sperm motility, morphology, and counts in the rat, rabbit, and dog: a consensus report. ILSI Risk Science Institute Expert Working Group on Sperm Evaluation. Reprod Toxicol. 1996; 10:237–244.
18. Gundersen HJ, Bagger P, Bendtsen TF, Evans SM, Korbo L, Marcussen N, Møller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sørensen FB, Vesterby A, West MJ. The new stereological tools: disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. APMIS. 1988; 96:857–881.
19. Karlsson LM, Cruz-Orive LM. Estimation of mean particle size from single sections. J Microsc. 1997; 186:121–132.
20. de Rooij DG, Vergouwen RP. The estimation of damage to testicular cell lineages. Prog Clin Biol Res. 1991; 372:467–480.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


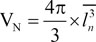
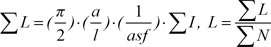

 XML Download
XML Download