Abstract
We found the changed distribution of glucose transporter (GLUT) proteins in the skin during rat development. At 15 days of gestation, GLUT1 and 2 proteins were expressed in the stratum corneum of epidermal cells. In postnatal skin, however, GLUT1 and 2 exhibit different expression patterns. While GLUT1 expression becomes more restricted to the stratum basale with development, GLUT2 was found mainly in stratum spinosum and granulosum, but not being localized in the stratum basale at any stages of perinatal skin development. Considering all these, it can be speculated that each GLUT protein plays its specific role in different epidermal layers and that the glucose used in mammalian skin in utero could be originated from the amniotic fluid during skin development.
Sugar, as a major energy component to all living cells, traverses lipid bilayer membranes through carrier-mediated transport [1]. Glycoproteins termed glucose transporter (GLUT) play an important role in the transport of glucose across the cytoplasmic membrane [1234]. GLUT family is composed of multiple member proteins; and each protein is expressed in different organs, showing a kind of tissue and organ specificity [5].
In fact, skin is a unique organ in terms of metabolism. As the epidermal cells in the skin are not directly vascularized, nutrient and oxygen are transported from dermis by a simple diffusion [6]. This means that study on GLUT protein could be very suggestive for complete comprehension of glucose transport mechanism in skin. Actually, pioneering studies already exhibited that GULT proteins are expressed in the skin epidermis [46789]. Among them, Davies et al. [7] and Kuroki et al. [8] showed that prickle cell, basal cells and the bulge region of hair follicles of the skin were immunostained by GLUT1 antibody.
Even so, morphological studies performed on the GLUT protein are not yet sufficient. Especially as for developing skin, the microscopic information is still absent. Considering that GLUT proteins have related with a skin keratinocyte differentiation [4], insufficient data about the proteins inhibit us from comprehending a complete role of GLUT in developing skin. In this study, we therefore tried to morphologically characterize the skin cells expressing GLUT1, 2, and 3 proteins at each developmental stage of rat skin.
To study the distribution of GLUT proteins in the rat epidermis at each developmental stage, Sprague-Dawley rats (n=30) were used in this study. The animals were treated in accordance with ‘The Guide for the Care and Use of Laboratory Animals’ (NIH publication No. 86-23, 1985 edition). The Institutional Animal Care and Use Committee (IACUC) of Seoul National University also approved this study (SNU-130508-3).
Animals were sacrificed by etherization and perfused through the heart with 4% paraformaldehyde. The skin tissues were removed after perfusion and placed overnight in the same fixative at 4℃. They were then washed three times in cold sodium phosphate buffer (0.1 M, pH 7.6) and were cryoprotected in sucrose gradient (10%, 20%, and 30% for 1.5–2 hours; and then in 30% sucrose overnight). Tissues were then embedded in optimal cutting temperature compound to be frozen rapidly in 2-methylbutane pre-cooled to its freezing point with liquid nitrogen. Tissue specimens were cut into 12 µm sections on the cryostat (Leica, Jena, Germany), thaw-mounted on gelatin-coated microscopic slides, and were stored at −20℃.
Immunohistochemistry was performed on the skin sections. The primary antibodies used were against mouse GLUT1 (ab652, Abcam, Cambridge, UK), GLUT2 (ab54460, Abcam), and GLUT3 (ab41525, Abcam). We also used Alexa Fluor 555 donkey anti-rabbit IgG (A31572, Invitrogen, Carlsbad, CA, USA) as a secondary antibody.
Prior to immunohistochemical staining, the sections were washed three times in cold phosphate buffered saline (PBS; 0.1 M, pH 7.4). They were then incubated sequentially in primary antibody (1:200 in 0.2% Triton X-100 buffer containing 0.1 M PBS) overnight at 4℃, and then in secondary antibody (1:200 in PBS) for 1 hour at room temperature. 4′,6-diamidino-2-phenylindole (DAPI; D1306, Molecular Probes, Eugene, OR, USA; 0.5 µg/ml in PBS) staining was done on the same slides for 30 minutes at room temperature. The stained slides were examined by fluorescence microscope (Olympus BX51, Tokyo, Japan).
In this study, GLUT1 and 2 showed intense immunoreactivity (IR) during skin development. At 15 days of gestation (G15), GLUT was observed in the epidermis that starts to be distinct from underlying dermis. At this stage, GLUT1 IR was found in most epidermal cells, including stratum corneum (Fig. 1). However, the GLUT1 expression in the epidermis was changed remarkably thereafter. GLUT1 was not detected diffusely in the epidermis any more, but became much confined to the stratum basale (basal layer). The pattern continued even at postnatal day 7 (P7) (Fig. 1). As for the bulged regions of hair follicle, in which Kuroki et al. [8] discovered GLUT1 IRs, we could not find any of GLUT1 IRs at P0 (Supplementary Fig. 1). However, from P7 on, GLUT1 IRs started to be observed at the bulged regions of hair follicles (Supplementary Fig. 2). As late as P70, GLUT1 expression pattern (Supplementary Fig. 3) was very similar to that reported in Kuroki et al.'s work [8] on the same protein.
On the other hand, GLUT2 shows different expression pattern in developing skin. Like GLUT1, GLUT2 was localized in stratum corneum at G15. At postnatal stage, however, GLUT2 IR was much intensely observed in strata spinosum and granulosum. Different from GLUT1, the postnatal cells of stratum basale did not express GLUT2 IR whereas the suprabasal cells of epidermis showed intense GLUT2 IRs at the same stage (Fig. 2). In case of GLUT3, however, we could not find any of significant IRs in the sections (Supplementary Fig. 4).
Previous studies showed that GLUT proteins in each organ and tissue show different patterns of expression [10]. While GLUT1 is ubiquitously expressed in various tissues and organs, GLUT2 is found much easily in specific organs such as liver, kidney, intestine, and pancreas [41112]. By our study, we can show the expression of GLUT proteins in developing skin for the first time ever. In previous study, cultured keratinocyte expressed GLUT1 and 2; and the differentiation of keratinocyte looked intensely associated with the changed expressions of GLUT1 and 2 [4]. In our immunohistochemical study on developing rat skin, we also found that each GLUT protein showed similar but unique expression patterns in developing rat skin.
First of all, both GLUT1 and 2 proteins are expressed in the stratum corneum at G15. Concerning this, we suspect if the sugar used by epidermal cells might not be exclusively transported from the dermis during embryonic period. Rather, the sugar might be transported from multiple sources, possibly also from amniotic fluid in utero.
To comprehend this, we note previous reports about the uptake of sugar occurring through the body surface. Briefly, in case of developing larvae of the parasitoid wasp, Aphidius ervi, they made alternative way of glucose transport for compensation (i.e., the nutrient absorption from epidermis) when the appearance of a functioning intestinal epithelium was delayed [13]. The authors speculated that A. ervi larva epithelium is presumably involved in nutrient absorption, at least during the earliest stages of development. Their hypothesis was evidenced further by the analysis with specific inhibitors, supporting the sugar transport role of GLUT2-like transporters in A. ervi larva epithelial cells [1314].
Considering the evolutionary conservation of GLUT proteins in glucose transport, similar trans-epithelial sugar transport might occur in the developing mammalian epidermis. As GLUT proteins are expressed in the apical cytoplasmic domain of epidermal cells at G18, they might play a role in transporting glucose from the amniotic fluid to skin during embryonic period. As the glucose transport through skin was known to decline rapidly with a maturation of A. ervi larva epithelium [13], weak GLUT expression in postnatal stratum corneum means a decrease in the glucose transport from amniotic fluid at later stage of skin development.
We also note that GLUT protein expression is remarkably changed after birth. In case of GLUT1, it was mainly observed in stratum basale at P7, and, to a lower extent, in the immediately suprabasal layer of the epidermis. The pattern is the same to the distribution of GLUT1 previously reported from adult human skin [6]. Meanwhile, it is also noteworthy that GLUT2 in post-natal skin showed different expression patterns from GLUT1. Whereas the post-natal stratum basale exhibited intense GLUT1 immunoreactivities, GLUT2 was not detected at all in the same basal layer, but were only confined to the suprabasal stratum spinosum and granulosum cells immediately adjacent to it. In short, based on our outcome, GLUT1 and 2 proteins might play a slightly different role in transporting glucose in postnatal epidermis. Glucose transport from dermis to epidermis can be done first by GLUT1 protein expressed in stratum basale, and then moved further into deep epidermis by the action of GLUT2 in the stratum spinosum and granulosum cells.
In addition, Shepard et al. [15] showed the glucose absorption and the changed concentration of amniotic glucose during rat development. They found that the concentration of amniotic glucose in developmental rat embryo was 27 ml/dl at 10 days of gestation, then decreased to 16.8 ml/dl at 20 days of gestation [15]. These data indicated that there may be the possibility of direct absorption and use of glucose from the amniotic fluids in the developmental rat embryo.
In this study, we can reveal that the GLUT proteins might play specific roles in different epidermal layer during skin development. While GLUT 1 expression shows a change with skin development, from entire epidermal layer to mainly a stratum basale, GLUT2 IRs were much diffusely distributed in stratum spinosum and granulosum, but not much localized in the stratum basale at any postnatal stages of skin development. The information can be suggestive for us to figure out more detailed function of GLUT proteins in developing mammalian skin.
Figures and Tables
Fig. 1
Glucose transporter (GLUT) 1 immunoreactivity (IR) at gestational day (G) 15, G18, postnatal day (P) 0 and P7. (A, D, G, J) Image of GLUT1 IRs. (B, E, H, K) Merged image of GLUT1 IRs and DAPI. (C, F, I, L) Magnified image of merged one. EP, epidermis; D, dermis. Asterisks indicate cells of stratum basale. At G15, GLUT1 IRs were found in most epidermal cells. In magnified image of G15, GLUT1 IRs were localized in every cytoplasmic domain (epical, lateral, and basal) of the cells. After G18, the GLUT1 expression was changed. GLUT1 IRs became mainly restricted to the stratum basale of epidermis. P0 and P7 images also showed the same pattern. Scale bars=40 µm (A–L).
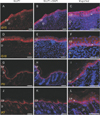
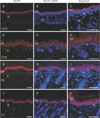
Fig. 2
Glucose transporter (GLUT) 2 immunoreactivity (IR) at gestational day (G) 15, G18, postnatal day (P) 0 and P7. (A, D, G, J) Image of GLUT2 IRs. (B, E, H, K) Merged image of GLUT2 IRs and DAPI. (C, F, I, L) Magnified image of merged one. EP, epidermis; D, dermis. Asterisks indicate cells of stratum basale. At G15, GLUT2 were localized in every cytoplasmic domain of most epidermal cells. In magnified image, the cells of stratum basale were not immune-stained by GLUT2 antibody. The absence of GLUT2 IRs in stratum basale continued even at G18, P0 and P7: the cells of stratum basale did not express GLUT2 IRs. Scale bars=40 µm (A–L).
Acknowledgements
This study was supported by the research grant (06-413) of the Asan Institute for Life Sciences, Asan Medical Center, Seoul, Korea, and by the SNUH research fund (grant number 04-2007-049).
References
1. Wilson-O'Brien AL, Patron N, Rogers S. Evolutionary ancestry and novel functions of the mammalian glucose transporter (GLUT) family. BMC Evol Biol. 2010; 10:152.
2. Gould GW, Holman GD. The glucose transporter family: structure, function and tissue-specific expression. Biochem J. 1993; 295(Pt 2):329–341.
3. Olson AL, Pessin JE. Structure, function, and regulation of the mammalian facilitative glucose transporter gene family. Annu Rev Nutr. 1996; 16:235–256.
4. Shen S, Wertheimer E, Sampson SR, Tennenbaum T. Characterization of glucose transport system in keratinocytes: insulin and IGF-1 differentially affect specific transporters. J Invest Dermatol. 2000; 115:949–954.
5. Joost HG, Bell GI, Best JD, Birnbaum MJ, Charron MJ, Chen YT, Doege H, James DE, Lodish HF, Moley KH, Moley JF, Mueckler M, Rogers S, Schürmann A, Seino S, Thorens B. Nomenclature of the GLUT/SLC2A family of sugar/polyol transport facilitators. Am J Physiol Endocrinol Metab. 2002; 282:E974–E976.
6. Gherzi R, Melioli G, de Luca M, D'Agostino A, Distefano G, Guastella M, D'Anna F, Franzi AT, Cancedda R. “HepG2/erythroid/brain” type glucose transporter (GLUT1) is highly expressed in human epidermis: keratinocyte differentiation affects GLUT1 levels in reconstituted epidermis. J Cell Physiol. 1992; 150:463–474.
7. Davies CA, Jeziorska M, Freemont AJ, Herrick AL. The differential expression of VEGF, VEGFR-2, and GLUT-1 proteins in disease subtypes of systemic sclerosis. Hum Pathol. 2006; 37:190–197.
8. Kuroki S, Yokoo S, Terashi H, Hasegawa M, Komori T. Epithelialization in oral mucous wound healing in terms of energy metabolism. Kobe J Med Sci. 2009; 55:E5–E15.
9. Tochio T, Tanaka H, Nakata S. Glucose transporter member 1 is involved in UVB-induced epidermal hyperplasia by enhancing proliferation in epidermal keratinocytes. Int J Dermatol. 2013; 52:300–308.
10. Bell GI, Burant CF, Takeda J, Gould GW. Structure and function of mammalian facilitative sugar transporters. J Biol Chem. 1993; 268:19161–19164.
11. Takata K. Glucose transporters in the transepithelial transport of glucose. J Electron Microsc (Tokyo). 1996; 45:275–284.
12. Wertheimer E, Sasson S, Cerasi E, Ben-Neriah Y. The ubiquitous glucose transporter GLUT-1 belongs to the glucose-regulated protein family of stress-inducible proteins. Proc Natl Acad Sci U S A. 1991; 88:2525–2529.
13. Giordana B, Milani A, Grimaldi A, Farneti R, Casartelli M, Ambrosecchio MR, Digilio MC, Leonardi MG, de Eguileor M, Pennacchio F. Absorption of sugars and amino acids by the epidermis of Aphidius ervi larvae. J Insect Physiol. 2003; 49:1115–1124.
14. de Eguileor M, Grimaldi A, Tettamanti G, Valvassori R, Leonardi MG, Giordana B, Tremblay E, Digilio MC, Pennacchio F. Larval anatomy and structure of absorbing epithelia in the aphid parasitoid Aphidius ervi Haliday (Hymenoptera, Braconidae). Arthropod Struct Dev. 2001; 30:27–37.
15. Shepard TH, Tanimura T, Park HW. Glucose absorption and utilization by rat embryos. Int J Dev Biol. 1997; 41:307–314.
Electronic Supplementary Materials
Supplementary data including four figures can be found with this article online at http://www.acbjournal.org/src/sm/acb-50-214-s001.pdf.
Supplementary Fig. 1
Magnified image of glucose transporter 1 (GLUT1) immunoreactivity in the developing rat skin at postnatal day 0 (P0).
Supplementary Fig. 2
Magnified image of glucose transporter 1 (GLUT1) immunoreactivity in the developing rat skin at postnatal day 7 (P7).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download