Abstract
4-Hydroxy-3-methoxybenzaldehyde (vanillin) and 4-hydroxybenzyl alcohol (4-HBA) are natural phenolic compounds, which present in many plants and have diverse biological properties. In this study, we examined effects of vanillin and 4-HBA on learning and memory function, cell proliferation, and neuroblast differentiation in the hippocampal dentate gyrus in a mouse model of scopolamine-induced amnesia. Scopolamine (SCO; 1 mg/kg/day, intraperitoneally), vanillin, and 4-HBA (40 mg/kg/day, orally) were administered for 28 days. Treatment with scopolamine alone impaired learning and memory function in the Morris water maze and passive avoidance tests, in addition, the treatment significantly reduced cell proliferation and neuroblast differentiation in the dentate gyrus, which were examined by immunohistochemistry for Ki-67 (a classic marker for cell proliferation) and doublecortin (a marker for neuroblasts). However, treatment with vanillin or 4-HBA significantly attenuated SCO-induced learning and memory impairment as well as the reduction of cell proliferation and neuroblast differentiation in the dentate gyrus. These results indicate that vanillin and 4-HBA may be helpful in improving cognitive function and in increasing endogenous neuronal proliferation in the brain.
Acetylcholine, the first neurotransmitter, is synthesized in cholinergic neurons in the brain by enzyme choline acetyltransferase and plays a crucial role for the regulation of multiple cognitive processes [12]. Abnormalities of the cholinergic system have been closely associated with a significant cognitive dysfunction [34].
Scopolamine (SCO), a competitive antagonist for muscarinic acetylcholine receptor, inhibits cholinergic transmission via blocking muscarinic acetylcholine receptor in the brain [5]. The hippocampus is known to show SCO-induced dysregulation of cholinergic system, which consequently leads to the impairment of hippocampal-dependent learning and memory [67]. Thus, SCO has been widely used to induce learning and memory impairment in rodent models of amnesia, which are useful in studying on cognitive impairments [89].
4-Hydroxy-3-methoxybenzaldehyde (vanillin) and 4-hydroxybenzyl alcohol (4-HBA) are well known as natural phenolic compounds which are found in diverse plants including Gastrodia elata Blume [1011]. These compounds have been received increasing interest due to their potential biological properties such as anti-tumor, anti-oxidant, and anti-inflammatory actions [12131415]. Additionally, with their high attention, many researchers have demonstrated potential neuroprotective properties of vanillin and 4-HBA against neuronal injury using in vitro and in vivo experiments. For example, Lee et al. [16] reported that vanillin and 4-HBA inhibited glutamate-induced apoptosis in human neuronal cells, and Kim et al. [17] proved that vanillin and 4-HBA protected hippocampal neurons from neuronal insult following transient global cerebral ischemia in gerbils. Furthermore, we recently reported that vanillin and 4-HBA promoted endogenous neurogenesis in the dentate gyrus of the hippocampus, which is well known to be affected in hippocampus-dependent learning and memory function [1819], in adolescent mice [20].
However, few studies regarding potential effects of vanillin and 4-HBA on cognitive function and endogenous neurogenesis in animal models of SCO-induced amnesia have been elucidated. Therefore, in this study, we examined effects of vanillin and 4-HBA on learning and memory function using the Morris water maze and passive avoidance tests and assessed changes in cell proliferation and neuroblast differentiation in the dentate gyrus of the hippocampus using immunohistochemistry for Ki-67 (a classic marker for cell proliferation) and doublecortin (a marker for neuroblast) in mice.
Male ICR mice (8 weeks of age; weight of 25–30 g) were purchased from the Orient Bio Inc. (Seoul, Korea) and handled and cared following the current international laws and policies (Guide for the Care and Use of Laboratory Animals, 8th edition, 2011) [21]. Experimental protocol in the present study was reviewed and approved based on ethical procedures and scientific care by the Kangwon National University-Institutional Animal Care and Use Committee (KW-160802-3).
Mice were randomly divided into four groups (n=14, per group) as follows: (1) vehicle (normal saline)-treated mice marked as “vehicle group,” (2) SCO (1 mg/kg/day)-treated mice marked as “SCO group,” (3) SCO (1 mg/kg/day) and vanillin (40 mg/kg/day)-treated mice marked as “SCO+vanillin group,” and (4) SCO (1 mg/kg/day) and 4-HBA (40 mg/kg/day)–treated mice marked as “SCO+4-HBA group.” SCO, vanillin, and 4-HBA were purchased from Sigma Aldrich (Sigma-Aldrich, St. Louis, MO, USA) and dissolved in normal saline. SCO was administered by intraperitoneal injection once daily for 4 weeks, and vanillin or 4-HBA were administered orally using a feeding needle once daily for 4 weeks. Experimental dosage of SCO, vanillin, and 4-HBA was selected on the basis of previous studies [2022].
Spatial learning and memory test was performed using the Morris water maze test according to a published procedure by Park et al. [22]. In short, a circular pool (90 cm in diameter and 45 cm height) filled with water was conceptually divided into four quadrants, and a platform (6 cm in diameter and 29 cm in height) was placed in one quadrant 1 cm below the water surface. Training was conducted for 3 consecutive days before the test, and the test was conducted on the last day. Mice were allowed to swim for 120 seconds to search for the hidden platform. If they failed to locate the platform within 120 seconds, escape would be assisted and escape latency was recorded as 120 seconds. At the end of each trial, each animal would stay on the platform for 3 seconds. After the training, the time required for individual animal to find the submerged platform within 120 seconds (escape latency) was recorded with Noldus Ethovision video tracking system (Ethovision XT, Noldus Information Technology, Wageningen, The Netherlands).
As previously described [23], short-term memory ability was evaluated by assessing the latency of the passive avoidance test. The test was performed with an apparatus that consisted of two compartments (light and dark) with a grid floor (GEM 392, San Diego Instruments, San Diego, CA, USA). The test was divided into a training session and a test session. In the training session, the door between the compartments was kept open, and animals were allowed to explore environments in the dark compartments for 2 minutes. Then lights were turned on in one compartment, and animals were allowed to explore environments in both light and dark compartments for 2 minutes. Then again, animals were given an inescapable foot shock (0.3 mA for 3 seconds) after entering the dark compartment by closing the door between the two compartments. The test session was performed 20 minutes after the training session using the same paradigm but without applying the foot shock. The interval between the start of the test session and their entry into the dark compartment was defined as the latency time of the passive avoidance test. When animals did not enter the dark room within 180 seconds, the latency was recorded as 180 seconds.
As previously described [22], in short, mice were anesthetized with sodium pentobarbital (30 mg/kg) and fixed with 4% paraformaldehyde. Their brains were removed and serially sectioned into 30-µm coronal sections in a cryostat (Leica, Wetzlar, Germany).
To examine neuronal death in the hippocampus after SCO treatment, Fluoro-Jade B (F-J B; a high-affinity fluorescent marker for neuronal degeneration) histofluorescence staining was conducted according to our published protocol [24]. In brief, the sections were first immersed in a solution containing 1% sodium hydroxide, transferred to a solution of 0.06% potassium permanganate, and transferred to a 0.0004% F-J B (Histochem, Jefferson, AR, USA) staining solution. Finally, the stained sections were observed using an epifluorescent microscope (Carl Zeiss, Jena, Germany) with blue (450–490 nm) excitation light and a barrier filter.
As previously described [25], immunohistochemical staining for neuronal nuclear antigen (NeuN, a marker for neurons), Ki-67 (a marker for proliferating cell) and doublecortin (DCX, a marker for neuroblast) was performed using rabbit anti-NeuN (1:1,000, Chemicon International, Temecula, CA, USA), rabbit anti-Ki-67 (1:100, Abcam, Cambridge, UK), or goat anti-DCX (1:100, Santa Cruz Biotechnology, Santa Cruz, CA, USA) as primary antibodies, and biotinylated goat anti-rabbit or rabbit anti-goat immunoglobulin G (1:200, Vector Laboratories, Burlingame, CA, USA) and streptavidin peroxidase complex (1:200, Vector Laboratories) as secondary antibodies. Finally, the antibodies were visualized with 3,3′-diaminobenzidine tetrahydrochloride. In order to establish the specificity of the immunostaining, a negative control test was carried out with pre-immune serum instead of primary antibody. The negative control resulted in the absence of immunoreactivity in all structures.
Quantitative analysis of the number of Ki-67– and DCX-immunoreactive cells was performed to our published procedure [24]. Briefly, images of all Ki-67– and DCX-immunoreactive structures were captured using a light microscope (BX53, Olympus, Hamburg, Germany) equipped with a digital camera (DP72, Olympus) connected to a personal computer monitor. The total number of Ki-67– or DCX-immunoreactive cells in all groups were counted in five sections/animal, which were selected with 120 µm interval between −1.46 to −2.46 mm posterior to the bregma in reference to a mouse atlas [26], using an Image Analysis System equipped with a computer-based CCD camera (Optimas 6.5, Cyber Metrics, Scottsdale, AZ, USA). The cell counts were obtained by averaging the counts from the tissue sections obtained from each animal.
The data shown here represent the means±SEM. The normality test was performed using Kolmogorov and Smirnov test for testing normal distributions, and Bartlett test for testing identical standard distributions. All data passed normality test. The differences among the means were statistically analyzed by a one-way analysis of variance followed by a Tukey's multiple range method to elucidate difference among the groups. Statistical analysis was performed using GraphPad Instat (Instat Statistics, GraphPad Software, La Jolla, CA, USA) and statistical significance was considered at P<0.05.
In the Morris water maze test, there was a significant increase in escape latency time in the SCO group compared with the vehicle group. However, in both SCO+vanillin and SCO+4-HBA groups, escape latency time was significantly decreased compared with that in the SCO group, and no significant difference was found in the escape latency time between these two groups (Fig. 1A).
In the SCO group, latency time in the passive avoidance test was significantly decreased compared with the vehicle group. However, latency time was significantly reversed in both SCO+vanillin and SCO+4-HBA groups compared with that in the SCO group; there was no significant difference in latency time between these two groups (Fig. 1B).
In the vehicle group, cells stained by Ki-67 antibody were found in the subgranular zone of the dentate gyrus (Fig. 2A). In the SCO group, a few Ki-67–immunoreactive cells were observed in the subgranular zone; the mean number of the cells was 3±0.6 cells (Fig. 2B, E). However, in both SCO+vanillin and SCO+4-HBA groups, the mean number of Ki-76–immunoreactive cells (8±0.9 and 8±1.1 cells, respectively) was significantly increased in the subgranular zone compared with that in the SCO group (Fig. 2C–E).
In the vehicle group, DCX-immunoreactive cells were easily found in the subgranular zone of the dentate gyrus. They showed well-developed (tertiary) dendrites that projected into the inner molecular layer (Fig. 3A, B). In the SCO group, the mean number of DCX-immunoreactive cells (23±2.2 cells) was significantly decreased in the subgranular zone, and they had very short processes compared to those in the vehicle group (Fig. 3C, D, I). Whereas in both SCO+vanillin and SCO+4-HBA groups, the mean number of DCX-immunoreactive cells (42±2.8 and 44±3.4 cells, respectively) was significantly increased in the subgranular zone, and their dendrites were longer and thicker than those in the SCO group (Fig. 3E–I).
In the vehicle group, NeuN-immunoreactive neurons were abundantly found in the dentate gyrus, especially, in the granule cell layer and polymorphic layer (Fig. 4A). In the SCO group, the distribution pattern of NeuN-immunoreactive neurons was similar to that in the vehicle group (Fig. 4C). In both SCO+vanillin and SCO+4-HBA groups, no significant difference in the distribution pattern of NeuN-immunoreactive neurons was found (Fig. 4E, G). In addition, in all experimental groups, F-J B–positive cells were not observed in the dentate gyrus (Fig. 4B, D, F, H). These results were consistent with the findings from previous studies [2224].
Cognitive impairment following SCO treatment in rodents has been well established by various behavioral tasks used to assess learning and memory, and rodent models of SCO-induced amnesia have been widely used to investigate the efficacy of agents which have possibility to improve cognitive deficits [827].
In this study, we examined effects of vanillin and 4-HBA on cognitive impairment induced by chronic treatment with SCO in mice using the Morris water maze and passive avoidance behavior tests. In the Morris water maze test, SCO-treated mice significantly required much more time to find the platform hidden than that in the vehicle group, and vanillin or 4-HBA–treated mice in combination with SCO easily found the location of platform. In addition, in the passive avoidance test, treatment with vanillin or 4-HBA effectively restored the latency time which was reduced by chronic SCO treatment. Previous studies in vivo reported that vanillin and 4-HBA have protective effects against cognitive impairment. For instance, the administration of vanillin attenuated learning and memory impairment induced by chronic cerebral hypoperfusion-induced vascular dementia and 3-nitropropionic acid-induced Huntington's disease in mice and rats, which was assessed by the Morris water maze test [2829]. In addition, Hsieh et al. [30] reported that 4-HBA improved cycloheximide- and apomorphine-induced memory impairment in mice, which was tested by a passive avoidance test. Thus, on the basis of these reports as well as our result, we suggests that vanillin and 4-HBA can ameliorate SCO-induced learning and memory deficits in mice.
The hippocampus is a major component of the brain and is responsible for regulating cognitive function such as learning and memory, and reasoning [3132]. It has been reported that newly generated neurons in the dentate gyrus of the hippocampus play critical roles in learning and memory function and that a decline in hippocampal neurogenesis displays a negative effect on learning and memory [1833]. We reported that chronic treatment with SCO led to a significant reduction of endogenous neurogenesis without any neuronal loss in the dentate gyrus in mice [2434]. Furthermore, we recently reported that vanillin and 4-HBA effectively promoted hippocampal neurogenesis in normal mice [20]. On the basis of our previous studies, in the present study, we first examined whether vanillin and 4-HBA ameliorated SCO-induced reduction in neurogenesis in the dentate gyrus using immunohistochemistry for Ki-67 and DCX, which has been widely used as alternative endogenous markers for adult neurogenesis [3536], and we found that the treatment with vanillin or 4-HBA significantly increased numbers of Ki-67– and DCX-immunoreactive cells in the mouse dentate gyrus compared with the SCO group. In addition, DCX-immunoreactive cells in the vanillin or 4-HBA–treated group showed a well-developed dendritic complexity compared with the SCO group. Previous studies have reported that complexity of neuroblast dendrites is related to survival of newly generated neurons and synaptic connection of granule cells in the hippocampal dentate gyrus [3738]. Therefore, this result indicates that vanillin and 4-HBA can reverse the SCO-induced decrease of cell proliferation and neuroblast differentiation in the hippocampal dentate gyrus, and we suggest that this effect may be associated with the amelioration of the SCO-induced learning and memory impairment following vanillin and 4-HBA treatment.
In summary, we demonstrated that vanillin and 4-HBA effectively attenuated learning and memory impairment and a reduction of cell proliferation and neuroblast differentiation in the hippocampal dentate gyrus in a mouse model of SCO-induced amnesia. These findings indicate that vanillin and 4-HBA can be used as potential candidates for improving impaired cognitive function and neurogenesis, although further studies need to clearly understand the underlying mechanisms regarding effects of vanillin and 4-HBA against SCO-induced amnesia.
Figures and Tables
 | Fig. 1The latency of Morris water maze (A) and passive avoidance tests (B) in the vehicle, scopolamine (SCO), SCO+vanillin, and SCO+4-hydroxybenzyl alcohol (4-HBA) groups (n=7 per group in each test; *P<0.05, significantly different from the vehicle group, #P<0.05, significantly different from the SCO group). The bars indicate the means±SEM. |
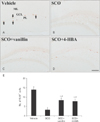 | Fig. 2Immunohistochemistry for Ki-67 in the dentate gyrus of the vehicle (A), scopolamine (SCO) (B), SCO+ vanillin (C), and SCO+4-hydroxybenzyl alcohol (4-HBA) (D) groups. In the vehicle group, Ki-67–immunoreactive cells (arrows) are shown in the subgranular zone. Ki-67-immunoreactive cells are significantly decreased in the SCO group; however, they are increased in the SCO+vanillin and SCO+4-HBA groups. GCL, granule cell layer; ML, molecular layer; PL, polymorphic layer. Scale bar=100 µm. (E) The mean number of Ki-67–immunoreactive cells per group (n=14 per group; *P<0.05, significantly different from the vehicle group, #P<0.05, significantly different from the SCO group). The bars indicate the means±SEM. |
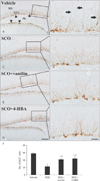 | Fig. 3Immunohistochemistry for doublecortin (DCX) in the dentate gyrus of the vehicle (A, B), scopolamine (SCO) (C, D), SCO+vanillin (E, F), and SCO+4-hydroxybenzyl alcohol (4-HBA) (G, H) groups. In the vehicle group, DCX-immunoreactive cells (arrowheads) have well-developed dendrites that extend into the molecular layer (ML, arrows). A few DCX-immunoreactive cells with short dendrites are found in the SCO group; however, numbers of DCX-immunoreactive cells and their dendrites are reversed in both SCO+vanillin and SCO+4-HBA group. GCL, granule cell layer; PL, polymorphic layer. Scale bars=100 (A, C, E, G) µm, 50 (B, D, F, H) µm. (I) The mean number of DCX-immunoreactive cells per group (n=14 per group; *P<0.05, significantly different from the vehicle group, #P<0.05, significantly different from the SCO group). The bars indicate the means± SEM. |
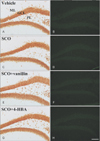 | Fig. 4NeuN immunohistochemistry (A, C, E, G) and Fluoro-Jade B (F-J B) histofluorescence staining (B, D, F, H) in the dentate gyrus of the vehicle (A, B), scopolamine (SCO) (C, D), SCO+vanillin (E, F), and SCO+4-hydroxybenzyl alcohol (4-HBA) (G, H) groups. There is no difference in the distribution pattern of NeuN-immunoreactive neurons between all groups, and no F-J B–positive cells are detected in all groups. GCL, granule cell layer; ML, molecular layer; PL, polymorphic layer. Scale bar=100 µm. The bars indicate the means±SEM. |
Acknowledgements
This work was supported by a Priority Research Centers Program grant (NRF-2009-0093812) through the National Research Foundation of Korea funded by the Ministry of Science, ICT and Future Planning.
References
1. Blokland A. Acetylcholine: a neurotransmitter for learning and memory? Brain Res Brain Res Rev. 1995; 21:285–300.
2. Blusztajn JK, Wurtman RJ. Choline and cholinergic neurons. Science. 1983; 221:614–620.
3. Bartus RT, Dean RL 3rd, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982; 217:408–414.
4. Mohapel P, Leanza G, Kokaia M, Lindvall O. Forebrain acetylcholine regulates adult hippocampal neurogenesis and learning. Neurobiol Aging. 2005; 26:939–946.
5. Falsafi SK, Deli A, Höger H, Pollak A, Lubec G. Scopolamine administration modulates muscarinic, nicotinic and NMDA receptor systems. PLoS One. 2012; 7:e32082.
6. Abe E. Reversal effect of DM-9384 on scopolamine-induced acetylcholine depletion in certain regions of the mouse brain. Psychopharmacology (Berl). 1991; 105:310–316.
7. Lee S, Kim J, Seo SG, Choi BR, Han JS, Lee KW, Kim J. Sulforaphane alleviates scopolamine-induced memory impairment in mice. Pharmacol Res. 2014; 85:23–32.
8. Cunha GM, Canas PM, Melo CS, Hockemeyer J, Müller CE, Oliveira CR, Cunha RA. Adenosine A2A receptor blockade prevents memory dysfunction caused by beta-amyloid peptides but not by scopolamine or MK-801. Exp Neurol. 2008; 210:776–781.
9. Manral A, Meena P, Saini V, Siraj F, Shalini S, Tiwari M. DADS analogues ameliorated the cognitive impairments of Alzheimer-like rat model induced by scopolamine. Neurotox Res. 2016; 30:407–426.
10. Cao Y, Zhang X, Fang Y, Ye J. Determination of the active ingredients in Gastrodia rhizoma by capillary electrophoresis with electrochemical detection. Analyst. 2001; 126:1524–1528.
11. Yang XD, Zhu J, Yang R, Liu JP, Li L, Zhang HB. Phenolic constituents from the rhizomes of Gastrodia elata. Nat Prod Res. 2007; 21:180–186.
12. Jung TY, Suh SI, Lee H, Kim IS, Kim HJ, Yoo HS, Lee SR. Protective effects of several components of Gastrodia elata on lipid peroxidation in gerbil brain homogenates. Phytother Res. 2007; 21:960–964.
13. Kim NH, Xin MJ, Cha JY, Ji SJ, Kwon SU, Jee HK, Park MR, Park YS, Kim CT, Kim DK, Lee YM. Antitumor and immunomodulatory effect of Gastrodia elata on colon cancer in vitro and In vivo. Am J Chin Med. 2017; 45:319–335.
14. Lee JY, Jang YW, Kang HS, Moon H, Sim SS, Kim CJ. Anti-inflammatory action of phenolic compounds from Gastrodia elata root. Arch Pharm Res. 2006; 29:849–858.
15. Lirdprapamongkol K, Sakurai H, Kawasaki N, Choo MK, Saitoh Y, Aozuka Y, Singhirunnusorn P, Ruchirawat S, Svasti J, Saiki I. Vanillin suppresses in vitro invasion and in vivo metastasis of mouse breast cancer cells. Eur J Pharm Sci. 2005; 25:57–65.
16. Lee YS, Ha JH, Yong CS, Lee DU, Huh K, Kang YS, Lee SH, Jung MW, Kim JA. Inhibitory effects of constituents of Gastrodia elata Bl. on glutamate-induced apoptosis in IMR-32 human neuroblastoma cells. Arch Pharm Res. 1999; 22:404–409.
17. Kim HJ, Hwang IK, Won MH. Vanillin, 4-hydroxybenzyl aldehyde and 4-hydroxybenzyl alcohol prevent hippocampal CA1 cell death following global ischemia. Brain Res. 2007; 1181:130–141.
18. Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010; 11:339–350.
19. Gould E, Tanapat P, Hastings NB, Shors TJ. Neurogenesis in adulthood: a possible role in learning. Trends Cogn Sci. 1999; 3:186–192.
20. Cho JH, Park JH, Ahn JH, Lee JC, Hwang IK, Park SM, Ahn JY, Kim DW, Cho JH, Kim JD, Kim YM, Won MH, Kang IJ. Vanillin and 4-hydroxybenzyl alcohol promotes cell proliferation and neuroblast differentiation in the dentate gyrus of mice via the increase of brain-derived neurotrophic factor and tropomyosin-related kinase B. Mol Med Rep. 2016; 13:2949–2956.
21. Institute of Laboratory Animal Research. Committee for the Update of the Guide for the Care and Use of Laboratory Animals. National Research Council. Guide for the care and use of laboratory animals. 8th ed. Washington, DC: National Academies Press;2011. p. 220.
22. Park JH, Choi HY, Cho JH, Kim IH, Lee TK, Lee JC, Won MH, Chen BH, Shin BN, Ahn JH, Tae HJ, Choi JH, Chung JY, Lee CH, Kang IJ, Kim JD. Effects of chronic scopolamine treatment on cognitive impairments and myelin basic protein expression in the mouse hippocampus. J Mol Neurosci. 2016; 59:579–589.
23. Lee JC, Park JH, Ahn JH, Kim IH, Cho JH, Choi JH, Yoo KY, Lee CH, Hwang IK, Cho JH, Kwon YG, Kim YM, Kang IJ, Won MH. New GABAergic neurogenesis in the hippocampal CA1 region of a gerbil model of long-term survival after transient cerebral ischemic injury. Brain Pathol. 2016; 26:581–592.
24. Yan BC, Park JH, Chen BH, Cho JH, Kim IH, Ahn JH, Lee JC, Hwang IK, Cho JH, Lee YL, Kang IJ, Won MH. Long-term administration of scopolamine interferes with nerve cell proliferation, differentiation and migration in adult mouse hippocampal dentate gyrus, but it does not induce cell death. Neural Regen Res. 2014; 9:1731–1739.
25. Yan BC, Kim IH, Park JH, Ahn JH, Cho JH, Chen BH, Lee JC, Choi JH, Yoo KY, Lee CH, Cho JH, Kim JD, Won MH. Systemic administration of low dosage of tetanus toxin decreases cell proliferation and neuroblast differentiation in the mouse hippocampal dentate gyrus. Lab Anim Res. 2013; 29:148–155.
26. Paxinos G, Franklin KB. The mouse brain in stereotaxic coordinates. San Diego, CA: Academic Press;1997.
27. Vasileva LV, Getova DP, Doncheva ND, Marchev AS, Georgiev MI. Beneficial effect of commercial Rhodiola extract in rats with scopolamine-induced memory impairment on active avoidance. J Ethnopharmacol. 2016; 193:586–591.
28. Gupta S, Sharma B. Pharmacological benefits of agomelatine and vanillin in experimental model of Huntington's disease. Pharmacol Biochem Behav. 2014; 122:122–135.
29. Gupta S, Sharma B, Singh P, Sharma BM. Modulation of transient receptor potential vanilloid subtype 1 (TRPV1) and norepinephrine transporters (NET) protect against oxidative stress, cellular injury, and vascular dementia. Curr Neurovasc Res. 2014; 11:94–106.
30. Hsieh MT, Wu CR, Chen CF. Gastrodin and p-hydroxybenzyl alcohol facilitate memory consolidation and retrieval, but not acquisition, on the passive avoidance task in rats. J Ethnopharmacol. 1997; 56:45–54.
31. Goel V, Makale M, Grafman J. The hippocampal system mediates logical reasoning about familiar spatial environments. J Cogn Neurosci. 2004; 16:654–664.
32. Jarrard LE. On the role of the hippocampus in learning and memory in the rat. Behav Neural Biol. 1993; 60:9–26.
33. Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001; 410:372–376.
34. Yoo DY, Kim W, Yoo KY, Lee CH, Choi JH, Kang IJ, Yoon YS, Kim DW, Won MH, Hwang IK. Effects of Nelumbo nucifera rhizome extract on cell proliferation and neuroblast differentiation in the hippocampal dentate gyrus in a scopolamine-induced amnesia animal model. Phytother Res. 2011; 25:809–815.
35. Couillard-Despres S, Winner B, Schaubeck S, Aigner R, Vroemen M, Weidner N, Bogdahn U, Winkler J, Kuhn HG, Aigner L. Doublecortin expression levels in adult brain reflect neurogenesis. Eur J Neurosci. 2005; 21:1–14.
36. Kee N, Sivalingam S, Boonstra R, Wojtowicz JM. The utility of Ki-67 and BrdU as proliferative markers of adult neurogenesis. J Neurosci Methods. 2002; 115:97–105.
37. Plumpe T, Ehninger D, Steiner B, Klempin F, Jessberger S, Brandt M, Römer B, Rodriguez GR, Kronenberg G, Kempermann G. Variability of doublecortin-associated dendrite maturation in adult hippocampal neurogenesis is independent of the regulation of precursor cell proliferation. BMC Neurosci. 2006; 7:77.
38. Ramirez-Rodriguez G, Ortíz-López L, Domínguez-Alonso A, Benítez-King GA, Kempermann G. Chronic treatment with melatonin stimulates dendrite maturation and complexity in adult hippocampal neurogenesis of mice. J Pineal Res. 2011; 50:29–37.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download