Abstract
In the present study, we examined change of ionized calcium-binding adapter molecule 1 (Iba-1) in the adult and aged gerbil spinal cords. Significant change of morphological feature and neuronal cell loss were not observed in both adult and aged spinal cords of gerbil after NeuN immunohistochemistry and Fluoro-Jade B histofluoresce staining. Iba-1–immunoreactive microglia broadly distributed in the spinal cord. Most of Iba-1–immunoreactive microglia showed ramified forms in the adult gerbil cervical and lumbar spinal cords. However, morphological changes of Iba-1–immunoreactive microglia were observed in the cervical and lumbar regions of the aged gerbil spinal cord. These microglia were showed a hypertrophied body with shortened swollen processes which was characteristic of activated microglia. In addition, Iba-1 protein level significantly higher in aged cervical and lumbar spinal cords than those in the adult gerbil. The present study showed an increase of activated forms of Iba-1–immunoreactive microglia and its protein level without marked changes in morphological features and neuronal loss in the aged spinal cord compared to those in the adult gerbil spinal cord. This result suggests that the increase of Iba-1 expression in the aged spinal cord may be closely associated with age-related changes in aged gerbil spinal cord.
Slow and progressive changes in the central nervous system (CNS) are characteristics in aging, and are closely associated with functional and morphological changes in the aged CNS. Among the various causes of aging, age-related changes of the immune system appears to play an important role in the processes of aging [1]. It has reported that aging is related closely to inflammation in the spinal cord [23]. Neurofibrillary pathological changes in the spinal cord were also reported in Alzheimer mouse model as well as in Alzheimer patients [45]. Microglia, a type of glial cells, act as the first and main form of active immune defense in the CNS [6]. They are broadly distributed in the parenchyma of the CNS. It is well known morphological microglial types such as ramified or resting, activated and amoeboid forms and microglia are very sensitive to even mild pathological changes. The ramified microglia are referred as a normal or resting state of microglia and they are transformed into activated or amoeboid forms of microglia under the pathological condition in the CNS [78].
Ionized calcium-binding adapter molecule 1 (Iba-1) is a calcium-binding protein that plays an important role in functional change of microglia [8910]. Iba-1 is widely used as a specific marker for all forms of microglia in the CNS as well as other organs. Many previous studies reported that up-regulation of Iba-1 in activated microglia under immune responses in the CNS [1112].
Mongolian gerbil is a good animal model for aging and ischemic experiments because of its brain vasculature and genetic characteristics [1314151617]. In addition, it was reported that gerbil could be used to study the role of substance P in neuropathic pain, because neurokinin 1 receptors in gerbils is comparable to those in humans [1819]. Although, some recent studies have reported age-related increase in the number of activated microglia in mice and rats, there was no comparative study on age-related changes in Iba-1 in the gerbil spinal cord. Therefore, in this study, we investigated differences of Iba-1 expression and its protein level between the adult and aged gerbil spinal cord.
Male Mongolian gerbils (Meriones unguiculatus) were used in this study. Postnatal month (PM) 6 (n=12) gerbils for adult group PM 24 (n=12) gerbils for aged group were housed in a conventional state under adequate temperature (23℃) and humidity (60%) control with a 12-hour light/12-hour dark cycle, and free access to food and water. The procedures for handling and caring for the animals adhered to the guidelines that are in compliance with the current international laws and policies (NIH Guide for the Care and Use of Laboratory Animals, NIH Publication No. 85-23, 1985, revised 1996). All of the experiments were conducted to minimize the number of animals used and the suffering caused by the procedures used in the present study.
The animals (n=7) were anesthetized with Zoletil50 (8 mg/kg) and xylazine (2 mg/kg) mixture and perfused transcardially with 0.1 M phosphate bufferd saline (PBS; pH 7.4) followed by 4% paraformaldehyde in 0.1 M PB (pH 7.4) for histochemical study. The spinal cords were removed and postfixed in the same fixative for 4 hours. The cervical (C6-C8) and lumbar (L5-L6) spinal cord tissues were cryoprotected by infiltration with 30% sucrose overnight. Thereafter the frozen tissues were serially sectioned on a cryostat (Leica, Wetzlar, Germany) into 30 µm thickness.
Fluoro-Jade B (F-J B) histofluorescence staining procedures were conducted according to the method by previous study [20]. Briefly, the sections were first immersed in a solution containing 1% sodium hydroxide in 80% alcohol, and followed in 70% alcohol. They were then transferred to a solution of 0.06% potassium permanganate, and transferred to a 0.0004% F-J B (Histochem, Jefferson, AR, USA) staining solution. The sections were placed on a slide warmer (approximately 50℃), and then examined using an fluorescent microscope (BX53, Olympus, Tokyo, Japan) with blue (450–490 nm) excitation light and a barrier filter. With this method neurons that undergo degeneration brightly fluorescence in comparison to the background [16].
Immunohistochemistry for neuronal nuclei (NeuN) and Iba-1 was performed under the same conditions in gerbils of different ages and spinal cord levels in order to examine whether the degree of immunohistochemical staining was accurate. The sections were sequentially treated with 0.3% H2O2 in PBS and 10% normal goat serum in 0.05 M PBS. They were then incubated with diluted mouse anti-NeuN (diluted 1:500, Millipore, Darmstadt, Germany) or rabbit anti–Iba-1 (diluted 1:400, Wako, Tokyo, Japan) respectively and subsequently exposed to biotinylated goat anti-mouse or rabbit and streptavidin peroxidase complex (1:200, Vector, Burlingame, CA, USA). They were then visualized by staining with 3,3′-diaminobenzidine in 0.1 M Tris-HCl buffer (pH 7.2) and were mounted in Canada balsam (Kanto, Tokyo, Japan) following dehydration. Images of all NeuN-immunoreactive structures taken from the cervical and lumbar spinal cords were obtained through a microscope (BX53, Olympus) equipped with a digital camera (DP72, Olympus). For calculating the number of NeuN-immunoreactive cells, 30 sections with 180-µm interval per animal were selected in the cervical and lumbar spinal cords. The number of NeuN-immunoreactive neurons in each groups was counted using an image analyzing system equipped with a computer-based digital camera (software: Optimas 6.5, CyberMetrics, Scottsdale, AZ, USA) in 30 sections per animal. Cell counts were obtained by averaging the counts from the sections of each animal and data represent as the number per section.
Analysis of the Iba-1–immunoreactivity in the spinal cords was performed using an image analysis system. Images were calibrated into an array of 512×512 pixels. Each pixel resolution was 256 gray levels. The intensity of Iba-1 immunoreactivity was evaluated by relative optical density (ROD), which was obtained after transformation of the mean gray level using the formula: ROD=log(256/mean gray level). ROD of background was determined in unlabeled portions and this value was subtracted for correction, yielding high ROD values in the presence of preserved structures and low values after structural loss using ImageJ 1.50 software (National Institutes of Health, Bethesda, MD, USA). A ratio of the ROD was calibrated as percentage compared to corresponding adult groups.
To confirmed changes in Iba-1 protein level in the cervical, lumbar spinal cords, animals (n=5 per group) were sacrificed and spinal cord were removed for western blot analysis. The tissues were cut to 500 µm thickness in a vibratome (Leica) and were homogenized in 50 mM PBS (pH 7.4) containing 0.1 mM ethylene glycol-bis(2-aminoethyl ether)-N,N,N′,N′ tetraacetic acid (pH 8.0), 0.2% nonidet P-40, 10 mM ethylendiamine tetraacetic acid (pH 8.0), 15 mM sodium pyrophosphate, 100 mM β-glycerophosphate, 50 mM NaF, 150 mM NaCl, 2 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, and 1 mM dithiothreitol (DTT). After centrifugation, the protein level was determined in the supernatants using a Micro BCA protein assay kit with bovine serum albumin as the standard (Pierce Chemical, Rockford, IL, USA). Aliquots containing 20 µg of total protein were boiled in loading buffer containing 150 mM Tris (pH 6.8), 3 mM DTT, 6% sodium dodecyl sulfate, 0.3% bromophenol blue and 30% glycerol. Then, each aliquot were loaded onto a 15% polyacrylamide gel. After electrophoresis, the gels were transferred to nitrocellulose transfer membranes (Pall Crop, East Hills, NY, USA). To reduce background staining, the membranes were incubated with 5% non-fat dry milk in PBS containing 0.1% Tween 20 for 45 minutes, followed by incubation with rabbit anti–Iba-1 (diluted in 1:500, Wako), peroxidase-conjugated goat anti-rabbit IgG (Sigma, St. Louis, MO, USA) and an ECL kit (Pierce Chemical). The blot was scanned for quantification of the relative optical density of each band using ImageJ 1.59 software (National Institutes of Health).
To investigate structure and distribution of neurons in the cervical and lumbar levels of the spinal cord, NeuN (a neuronal marker) staining was performed in the adult and aged gerbils. NeuN-immunoreactive neurons were distributed throughout the gray matter of the spinal cord (Fig. 1A–D). The number of NeuN-immunoreactive neurons was not significantly different between groups (Fig. 1M).
To examine neuronal death in the spinal cord, F-J B (a useful marker for neuronal degeneration) staining was performed in the adult and aged spinal cords. F-J B positive cells were not observed in the cervical and lumbar levels of the spinal cord in the adult and aged gerbil (Fig. 1D–L).
Iba-1 immunoreaction was well detected in both gray and white matter in the cervical and lumbar spinal cord of the adult and aged gerbils (Figs. 2, 3). In the adult group, most of Iba-1–immunoreactive microglia had a small cell body with highly ramified processes: These cells were identified as ramified microglia based on their morphology (Fig. 2G). In addition, some Iba-1–immunoreactive microglia showed a hypertrophied cell body with shortened and swollen processes (Fig. 2H). They were identified as activated microglia based on their morphology.
In the aged group, Iba-1–immunoreactive ramified forms of microglia were easily found. However, many of Iba-1–immunoreactive microglia showed activated forms in both the lumbar and spinal cords of the aged group than the adult cords (Figs. 2, 3). Very scarcely, Iba-1–immunoractive microglia showed amoeboid forms that had a large round body with some punctum in the aged lumbar spinal cord (Fig. 3I). Iba-1–immunoreactivity of aged spinal cords increased significantly to 128.9% and 157.1% than those in the adult cervical and lumbar spinal cords, respectively.
Western blot was performed to investigate change of Iba-1 protein levels in the adult and aged cervical and lumbar spinal regions in the gerbil. The result showed that Iba-1 protein levels in both spinal regions of the aged group were higher than those in the adult group (Fig. 4).
In the present study, we observed no significant changes in morphology and neuronal loss after NeuN immunohistochemistry in the adult and aged gerbil spinal cord. It was reported that not marked loss of neurons in the aged spinal cords of dog [2122]. In addition, we hardly observed F-J B stained neuron in the both cervical and lumbar region of adult and aged spinal cords. Therefore, marked neuronal loss in aged cervical and lumbar spinal cord may not be occurring with aging. This result is supported by previous studies reported that no significant neuronal loss of spinal neurons in the aged mice and dog [221].
We found abundant Iba-1–immunoractive microglia in both adult and aged spinal cords and most of microglia had a small cell body with highly ramified processes which is a characteristic of ramified microglia in the gerbil spinal cord in the present study. These Iba-1–immunoreactive microglia showed distinct morphological changes in the cervical and lumbar regions in the aged gerbil spinal cord compared to those in the adult. They had a swelled cell body with thick and short process which is characteristic an activated form of microglia based on previous studies [723]. It is well known that ramified microglia are transformed into activated microglia and phagocytic microglia after some pathological events following with inflammatory in the CNS [2425]. The increase Iba-1–immunoreactive activated forms of microglia in the aged gerbil spinal cord could reflect that certain circumferential changes are ongoing with aging in aged gerbil spinal cord without distinct neuronal degeneration. In addition, Iba-1 immunoreactivity significantly increased in both cervical and lumbar regions of aged gerbil spinal cords compared to those in adult gerbil. We also found significant increases of Iba-1 protein levels in both cervical and lumbar spinal cords of aged gerbil compared to those in adult. This result is supported by previous studies reported that an increase of Iba-1 expression on activated microglia in the brain and spinal cord [81126].
In the present study, we found increases Iba-1–immunoroeactive microglia activation and its immunoreactivity and protein levels in aged gerbil spinal cord without significant change of neuronal numbers. It has reported that microglial activation was increased in the spinal cords without neuronal loss in mice and dogs [2227]. Age related degenerative process in the spinal cord is accompanied by changes in various factors such as neurotransmitters, hormones, oxidative stress, and inflammation [2829303132]. In addition, activated microglia had bidirectional function. It has reported that activated microglia contribute to neurodegenerative diseases via various cytotoxic molecules production [73334]. On the hand, activated microglia also play important roles in protection against various pathological conditions in the CNS [3536]. Therefore, it needs further study to elucidate functional roles of activated Iba-1-immunoreactive microglia in the aged spinal cord.
In conclusion, the present study showed that morphological changes of Iba-1–immunoreactive microglia and increase of its protein level without neuronal loss in the cervical and lumbar spinal cords of the aged gerbil compared to those in the adult gerbil. This result suggests that increase of activated Iba-1–immunoractive microglia and its protein level may be related with aging process in the gerbil spinal cord.
Figures and Tables
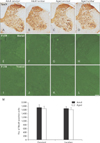 | Fig. 1Immunohistochemical staining for NeuN in the cervical and lumbar spinal cords of the adult (A, B) and aged (C, D) gerbils. Fluoro-Jade B (F-J B) histofluorescence staining for neurodegenerative neurons in the spinal cords of the adult (E, F, I, J) and aged (G, H, K, L) gerbils. Scale bar=200 µm (A–D), 80 µm (E–L). (M) The number of NeuN-immunoreactive neurons in the cervical and lumbar spinal cords per section. The bars indicate the means±SEM. |
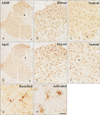 | Fig. 2Immunohistochemical staining for ionized calcium-binding adapter molecule 1 (Iba-1) in the cervical region of the adult (A–C) and aged (D, E) gerbils. High magnification photos of white boxes in panels (A) and (D) show in panels (B) to (C) and panels (E) to (F) with same character, respectively. (G, H) High magnification photos of white boxes in panels (C) and (F) show ramified and activated forms of Iba-1–immunoreactive microglia respectively. Scale bar=200 µm (A, D), 80 µm (B, C, E, F), 20 µm (G, H). |
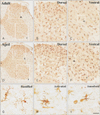 | Fig. 3Immunohistochemical staining for ionized calcium-binding adapter molecule 1 (Iba-1) in the lumbar region of the adult (A–C) and aged (D, E) spinal cords. High magnification photos of white boxes in panel (A) and (D) show in panels (B) to (C) and panels (E) to (F) with same character respectively. (G–I) High magnification photos of white boxes in panels (C) and (F) show ramified, activated and amoeboid forms of Iba-1–immunoreactive microglia respectively. Scale bar=200 µm (A, D), 80 µm (B, C, E, F), 20 µm (G, H). |
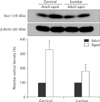 | Fig. 4Western blot analysis of ionized calcium-binding adapter molecule 1 (Iba-1) in the adult and aged spinal cords. Relative optical density of immunoblot band values are normalized to corresponding adult spinal region represents as percent (n=5 per group; *P<0.05, significantly different from the corresponding adult spinal region). The bars indicate the mean±SE. |
Acknowledgements
This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, No. NRF-2015R1D1A3A01020635).
References
1. Streit WJ, Xue QS. The brain's aging immune system. Aging Dis. 2010; 1:254–261.
2. Fu Y, Yu Y, Paxinos G, Watson C, Rusznák Z. Aging-dependent changes in the cellular composition of the mouse brain and spinal cord. Neuroscience. 2015; 290:406–420.
3. Zhang B, Bailey WM, McVicar AL, Gensel JC. Age increases reactive oxygen species production in macrophages and potentiates oxidative damage after spinal cord injury. Neurobiol Aging. 2016; 47:157–167.
4. Schmidt ML, Zhukareva V, Perl DP, Sheridan SK, Schuck T, Lee VM, Trojanowski JQ. Spinal cord neurofibrillary pathology in Alzheimer disease and Guam Parkinsonism-dementia complex. J Neuropathol Exp Neurol. 2001; 60:1075–1086.
5. Seo JS, Leem YH, Lee KW, Kim SW, Lee JK, Han PL. Severe motor neuron degeneration in the spinal cord of the Tg2576 mouse model of Alzheimer disease. J Alzheimers Dis. 2010; 21:263–276.
6. Ransohoff RM, Brown MA. Innate immunity in the central nervous system. J Clin Invest. 2012; 122:1164–1171.
7. Davis EJ, Foster TD, Thomas WE. Cellular forms and functions of brain microglia. Brain Res Bull. 1994; 34:73–78.
8. Ito D, Imai Y, Ohsawa K, Nakajima K, Fukuuchi Y, Kohsaka S. Microglia-specific localisation of a novel calcium binding protein, Iba1. Brain Res Mol Brain Res. 1998; 57:1–9.
9. Aloisi F. Immune function of microglia. Glia. 2001; 36:165–179.
10. Imai Y, Ibata I, Ito D, Ohsawa K, Kohsaka S. A novel gene Iba1 in the major histocompatibility complex class III region encoding an EF hand protein expressed in a monocytic lineage. Biochem Biophys Res Commun. 1996; 224:855–862.
11. Norden DM, Trojanowski PJ, Villanueva E, Navarro E, Godbout JP. Sequential activation of microglia and astrocyte cytokine expression precedes increased Iba-1 or GFAP immunoreactivity following systemic immune challenge. Glia. 2016; 64:300–316.
12. Ton BH, Chen Q, Gaina G, Tucureanu C, Georgescu A, Strungaru C, Flonta ML, Sah D, Ristoiu V. Activation profile of dorsal root ganglia Iba-1 (+) macrophages varies with the type of lesion in rats. Acta Histochem. 2013; 115:840–850.
13. Hwang IK, Yoo KY, Jung BK, Cho JH, Kim DH, Kang TC, Kwon YG, Kim YS, Won MH. Correlations between neuronal loss, decrease of memory, and decrease expression of brain-derived neurotrophic factor in the gerbil hippocampus during normal aging. Exp Neurol. 2006; 201:75–83.
14. Rich ST. The Mongolian gerbil (Meriones unguiculatus) in research. Lab Anim Care. 1968; 18:Suppl. 235–243.
15. Yoon DK, Hwang IK, Yoo KY, Lee YB, Lee JJ, Kim JH, Kang TC, Lee BH, Sohn HS, Won MH. Comparison of alpha-synuclein immunoreactivity and protein levels in ischemic hippocampal CA1 region between adult and aged gerbils and correlation with Cu,Zn-superoxide dismutase. Neurosci Res. 2006; 55:434–441.
16. Cheal ML. The gerbil: a unique model for research on aging. Exp Aging Res. 1986; 12:3–21.
17. Schmiedt RA. Effects of aging on potassium homeostasis and the endocochlear potential in the gerbil cochlea. Hear Res. 1996; 102:125–132.
18. Meert TF, Vissers K, Geenen F, Kontinen VK. Functional role of exogenous administration of substance P in chronic constriction injury model of neuropathic pain in gerbils. Pharmacol Biochem Behav. 2003; 76:17–25.
19. Lepre M, Evans RH, Olpe HR, Brugger F. The modulation of the monosynaptic reflex by substance P in the hemisected spinal cord preparation of the rat and gerbil. Neuroscience. 1993; 55:727–735.
20. Schmued LC, Hopkins KJ. Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000; 874:123–130.
21. Chung JY, Choi JH, Lee CH, Yoo KY, Won MH, Yoo DY, Kim DW, Choi SY, Youn HY, Moon SM, Hwang IK. Comparison of ionized calcium-binding adapter molecule 1-immunoreactive microglia in the spinal cord between young adult and aged dogs. Neurochem Res. 2010; 35:620–627.
22. Lee HJ, Choi JH, Ahn JH, Lee CH, Yoo KY, Hwang IK, Kim JS, Kim C, Lee YL, Shin HC, Won MH. Comparison of GAD65 and 67 immunoreactivity in the lumbar spinal cord between young adult and aged dogs. Neurochem Res. 2011; 36:435–442.
23. Streit WJ, Sammons NW, Kuhns AJ, Sparks DL. Dystrophic microglia in the aging human brain. Glia. 2004; 45:208–212.
24. Avignone E, Lepleux M, Angibaud J, Nägerl UV. Altered morphological dynamics of activated microglia after induction of status epilepticus. J Neuroinflammation. 2015; 12:202.
25. Korzhevskiĭ DE, Lentsman MV, Kirik OV, Otellin VA. Morphological types of activated microglia in the hippocampus observed following transient total brain ischemia. Morfologiia. 2012; 142:30–33.
26. Nakamura R, Nishimura T, Ochiai T, Nakada S, Nagatani M, Ogasawara H. Availability of a microglia and macrophage marker, iba-1, for differential diagnosis of spontaneous malignant reticuloses from astrocytomas in rats. J Toxicol Pathol. 2013; 26:55–60.
27. Ahn JH, Shin MC, Park JH, Kim IH, Lee JC, Yan BC, Hwang IK, Moon SM, Ahn JY, Ohk TG, Lee TH, Cho JH, Shin HC, Won MH. Increased immunoreactivity of cFos in the spinal cord of the aged mouse and dog. Mol Med Rep. 2015; 11:1043–1048.
28. Missale C, Govoni S, Castelletti L, Spano PF, Trabucchi M. Age related changes of enkephalin in rat spinal cord. Brain Res. 1983; 262:160–162.
29. Ranson RN, Dodds AL, Smith MJ, Santer RM, Watson AH. Age-associated changes in the monoaminergic innervation of rat lumbosacral spinal cord. Brain Res. 2003; 972:149–158.
30. Lozza FA, Chinchilla LA, Barbeito CG, Goya RG, Gimeno EJ, Portiansky EL. Changes in carbohydrate expression in the cervical spinal cord of rats during aging. Neuropathology. 2009; 29:258–262.
31. Tan H, He J, Wang S, Hirata K, Yang Z, Kuraoka A, Kawabuchi M. Age-related NADPH-diaphorase positive bodies in the lumbosacral spinal cord of aged rats. Arch Histol Cytol. 2006; 69:297–310.
32. Ahn JH, Choi JH, Kim JS, Lee HJ, Lee CH, Yoo KY, Hwang IK, Lee YL, Shin HC, Won MH. Comparison of immunoreactivities in 4-HNE and superoxide dismutases in the cervical and the lumbar spinal cord between adult and aged dogs. Exp Gerontol. 2011; 46:703–708.
33. Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996; 19:312–318.
34. Sheffield LG, Berman NE. Microglial expression of MHC class II increases in normal aging of nonhuman primates. Neurobiol Aging. 1998; 19:47–55.
35. Hailer NP, Jarhult JD, Nitsch R. Resting microglial cells in vitro: analysis of morphology and adhesion molecule expression in organotypic hippocampal slice cultures. Glia. 1996; 18:319–331.
36. Schwartz M, Butovsky O, Bruck W, Hanisch UK. Microglial phenotype: is the commitment reversible? Trends Neurosci. 2006; 29:68–74.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download