Abstract
The angiogenic theory to the development of human lymphatics is not clear. The objective of this study was to investigate the development of human lymphatics. Semi-thin and thin paraffin sections from human mature cystic ovarian teratoma tissues were studied using light and electron microscopy. Lymphatics were formed by the differentiation of mesenchymal cells that gradually acquired morphological features of endothelial cells. It is suggested that in human mature cystic ovarian teratoma the lymphatic endothelium develops from mesenchymal cells, and not from cells derived from mature endothelium of a preexisting vein or lymphatic.
The conventional concept of angiogenesis stated that endothelial cells initiate a process to uncover the basement membrane which later is digested by enzymes to leave some gaps. Through these gaps endothelial cells migrate and divide to form loops and tubes. A new basement membrane is then secreted and the open lumen of the new vessel communicates with that of the mother vessel [1]. Whether the same mechanism operates in human lymphatic development remains to be elucidated.
The choice of a suitable material for the study of human lymphatic development is difficult. In this study, surgically excised human mature cystic ovarian teratomas were chosen. Patients' consent and institutional review board permission were obtained.
Tissue blocks from solid areas of five specimens were excised and embedded in paraffin, 5 µm sections were mounted and stained with hematoxylin and eosin.
For electron microscopy, the tissues were fixed in 3% phosphate buffered glutaraldehyde and 1% osmium tetroxide, dehydrated in ascending ethanol, passed through propylene oxide, embedded in Spurr's resin and cut with an ultramicrotome. Lymphatics were identified in semi-thin sections and trimmed for ultra-thin sectioning, then stained with uranyl acetate and lead citrate and examined using a Jeol electron microscope (Tokyo, Japan), magnification ×100,000.
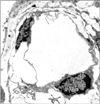
Areas of eosinophilic amorphous extracellular substance surrounded by several cells were observed. The nuclei had dispersed chromatin and did not appear to be at any stage of mitosis. The cytoplasm was scarce and extended in the form of cellular processes. Some processes were in conjunction with the cells lining the extracellular substance, while others projected away and formed a meshwork containing a less dense matrix. These findings were interpreted as a starting point of developing lymphatic capillaries lined by mesenchymal cells (Fig. 1).
In one section, the scalloped eosinophilic substance was surrounded by several cells similar to those shown in Fig. 1, but with thinner nuclei and less numerous cytoplasmic processes, especially those forming a meshwork nearby (Fig. 2). This vessel has been interpreted as a more developed lymphatic because of its continuous lining of mesenchymal cells.
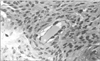
In a longitudinal section of a similar vessel, zones lined by slender cytoplasmic processes were intermingled with zones lined by a thicker cytoplasm harboring the cell nuclei. The outgoing cytoplasmic processes were fewer and shorter. The general appearance of a well formed lymphatic was observed in Fig. 3.
In the early stages of lymphatic vessel formation, the lining mesenchymal cells had large nuclei, scarce perinuclear cytoplasm and multiple thin cytoplasm processes and few organelles, as usual for undifferentiated cells.
In the more advanced stages of vessel formation, the lining cells showed microvesicles, in the form of caveolae or pinocytotic vesicles, overlapping intercellular junctions as well as tight junctions. The cytoplasm had more developed ribosomes and mitochondria. The nuclei were flatter and had a chromatin pattern similar to that of endothelial cells. Interdigitating junctions were only seen at a very late stage and only in fully developed lymphatics, which were scarce in this type of specimen (Fig. 4).
Light and electron microscopy describe “endothelial-like” structures in lymphatics [2]. This resemblance may mean that these cells look like endothelium but do belong to some other category of cells. This study suggests that this resemblance is simply because the cells are in a developmental stage, before differentiating into endothelial cells.
There is a consensus that lymphatics should be lined by endothelium. The present study investigated developing lymphatics, where typical endothelial lining was not always observed. Instead, the amorphous eosinophilic content was considered diagnostic.
Immunocytochemistry is widely used to detect specific surface antigens of endothelial cells. Utilizing this method, Conzonieri et al. [3] have shown focal expression of factor IX related antigen by putative endothelial cells in some cysts.
Klika et al. [4] found that mesenchymal cells form part of the wall of the primitive lumphatics of the epicardium of check embryo. Al-Jomard and Scothorne [5] demonstrated that lymphatics in rat liver develop from in-situ differentiation of mesenchymal cells into endothelial cells. These findings are further evidence to the conclusion of this study that the lymphatic endothelium in human mature cystic ovarian teratoma is of mesenchymal origin.
Figures and Tables
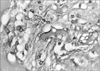
Fig. 1
Light micrograph showing two vessel-like structures, with a center of amorphous eosinophilic substance surrounded by mesenchymal cells lining the center and expanding slender cell processes in the vicinity (×400).
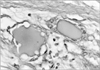
Fig. 2
Light micrograph showing a center of amorphous eosinophilic substance lined by several cells. Some cells have a thin profile creating a single opening while other cells have rounded profiles (×400).
References
1. Fajardo LF. The complexity of endothelial cells: a review. Am J Clin Pathol. 1989; 92:241–250.
2. Leak LV. The structure of lymphatic capillaries in lymph formation. Fed Proc. 1976; 35:1863–1871.
3. Canzonieri V, Volpe R, Gloghini A, Carbone A. Sieve-like areas in mature cystic teratomas of the ovary: a histochemical and immunohistochemical study of 7 cases. Pathologica. 1994; 86:43–46.
4. Klika E, Antaliková L, Rychter Z, Jelínek R. Inception and manner of development of the lymph vessels in the chick embryo heart. Lymphology. 1972; 5:137–148.
5. Al-Jomard RM, Scothorne RJ. The development of lymphatics in the rat liver. In : Proceedings of the Anatomical Society of Great Britain and Ireland; 1986 June; Leicester, UK. London: Taylor and Francis;1986. p. 40.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download