Abstract
Vitamin C is an essential micronutrient that affects immune responses. T cells are one of the main players in acquired immunity and have been reported to be influenced by in vivo vitamin C supplementation. Yet, the way by which T cells uptake vitamin C and what direct effects vitamin C exerts on the cells are not known. To elucidate, we isolated human peripheral blood T cells and analyzed the expression of sodium-dependent vitamin C transporters (SVCT). T cells were activated in vitro in the absence or presence of vitamin C, before or after activation. As results, human T cells expressed SVCT2, but not SVCT1, and the expression level increased following activation. Vitamin C added in the culture media generally did not affect T-cell behaviors following activation, such as proliferation, apoptosis, expression of CD25 and CD69, and interleukin 2 secretion, regardless whether it was added before or after activation. However, exceptionally, high concentration vitamin C, when it was added before activation, but not after activation, did exert toxic effects on cell activation with respect to the above-mentioned parameters. In conclusion, we showed the expression of SVCT2 in human T cells for the first time. Vitamin C exerted toxic effects, at least in vitro, when the concentration was high and when it was given before activation. These toxic effects are not thought to be via anti-oxidant effects of vitamin C.
Vitamin C is a micronutrient which functions as an important physiological antioxidant [1]. There are two forms of vitamin C. One is its reduced form, the ascorbic acid (AA), and the other its oxidized form, the dihydroascorbic acid (DHA). DHA is transported into cells via glucose transporters (GLUT) 1, 3, and 4 passively along the concentration gradient [23]. Meanwhile, AA is actively transported via sodium-dependent vitamin C transporters (SVCT1 and 2) at the expense of energy [4567].
Men cannot, contrary to other animals, synthesize vitamin C by themselves because they lack the final step enzyme L-gulonolactone oxidase in the synthetic process of vitamin C from glucose [8]. Thus, men have to be supplemented with extrinsic source of vitamin C for their lives. The daily requirement of vitamin C is 75–90 mg [9], and many investigators insist beneficial effects of even more dose, up to 1–10 g/day, defined as mega-dose [1011].
In a cell, vitamin C influences a variety of biological processes, one of which is immunological response. Effects of vitamin C on immune responses have been documented both in human and in experimental animals. For example, vitamin C supplementation increased non-specific IgA and IgM titers in human serum [12], and antigen-specific antibody titers in guinea pig [13], while reduced serum IgE level in chronic granulomatous disease [14]. Mega-dose vitamin C administration shifted the overall immune response towards Th1 [15], thus augmented cell-mediated immunity in a variety of conditions, both in human and experimental animals [1617].
Until now, exact mechanisms of the immunological effects of vitamin C have not been elucidated, especially in humans. In experimental animal models, such efforts have been consistently, but not so much frequently, carried out. Recently, we reported that mega-dose vitamin C modulated mouse immune responses primarily acting on dendritic cells (DCs), thus affecting T cells and B cells subsequently [1819]. In vitro vitamin C–treated DCs rendered the immune response towards Th1, secreting more interleukin-12 p70 (IL-12p70) and interleukin (IL)-15 by way of elevated phosphorylation of p38 mitogen-activated protein kinase and ERK1/2, and increased activation of nuclear factor κB (NF-κB) [1520].
Still, many reports still suggest a role of vitamin C in human T cells. For example, T cells from vitamin C–supplemented old and young men showed more proliferating capacity when stimulated in vitro [1521]. The fact that human lymphocytes accumulate much higher concentration, up to 80-fold, of vitamin C compared to that in serum [22] also suggests a certain role of vitamin C in these cells. In addition, because reactive oxygen species (ROS) are formed during T-cell activation and act as a second messenger [2324], it could be possible that vitamin C affects T-cell behaviors during activation as an antioxidant.
In the present study, we evaluated how human T cells uptake vitamin C, and whether they are influenced in their function by the presence of various concentrations of vitamin C in vitro.
Blood samples were drawn from 10 healthy donors aging between 20s and 40s under consent approved by Institutional Review Board of our institute (approval No. C-1208-149-424). Peripheral blood mononuclear cells were obtained by density gradient centrifugation using Ficoll-paque PLUS (1.077±0.001 g/ml, Amersham, GE Healthcare, Piscataway, NJ, USA). CD3+ T cells were isolated using negative selection pan T cell isolation kit II (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) following the manufacturer's instruction. Isolated T cells showed over 95% purity when they were analyzed by flow cytometry using anti–CD3-PE (Becton Dickinson, Franklin Lakes, NJ, USA) (data not shown).
T cells were cultured in RPMI 1640 (Welgene, Daegu, Korea) supplemented with 10% fetal bovine serum (Gibco, Gaithersburg, MD, USA), 1% penicillin/streptomycin, 1% GlutaMAX (Gibco), 1 mM sodium pyruvate (Gibco), and 1% non-essential amino acids (Gibco).
If necessary, T cells were activated with 10 ng/ml of phorbol-12-myristate-13-acetate (PMA; Calbiochem, San Diego, CA, USA) and 50 ng/ml ionomycin (Sigma, St. Louis, MO, USA) or dynabeads human T-activator CD3/CD28 (Invitrogen, Carlsbad, CA, USA).
Total RNA was extracted from human T cells using Trizol reagent (Invitrogen) following the instruction. cDNA was synthesized as usual and was amplified by polymerase chain reaction (PCR) for detection of SVCT expression. PCR was performed in a 20 µl volume using 1.5 mM MgCl2, 0.1 mM dNTPs, 8 pmole of each sense and antisense primers, and 1 units of Taq polymerase. Primers used were 5'-GCCCCTGAACACCTCTCATA-3' and 5'-ATGGCCAGCATGATAGGA AA-3' for human SVCT-1 (product size, 360 bp) [25], 5'-TTCTATGCTCG CACAGATGCC-3' and 5'-TAAAAGCCACACAGCCCCC TAC-3' for human SVCT-2 (product size, 667 bp) [26], and 5'-GTGGAGTCTACTGGCGTCTT-3', and 5'-GCCTGCTTC ACCACCTTCTT-3' for glyceraldehyde 3-phosphate dehydrogenase (GAPDH; product size, 509 bp). PCR for SVCT1 and SVCT2 was performed 40 cycles of denaturation at 95℃ for 45 seconds, annealing at 55℃ or 61℃ respectively for 45 seconds, and amplification at 72℃ at 45 seconds. For GAPDH PCR, 30 cycles were carried out with denaturation at 95℃ for 30 seconds, annealing at 58℃ for 30 seconds, and amplification at 72℃ for 30 seconds. The PCR products were analyzed by 2% agarose gel electrophoresis and subjected to densitometric analysis using Quantity One software (Bio-Rad Laboratories, Hercules, CA, USA).
Human T cells were lyzed in RIPA lysis buffer (50 mM Tris-HCl pH 7.4, 150 mM NaCl, 1% sodium deoxychloride, 0.1% sodium dodecyl sulfate, 1% Triton X-100, 2 mM EDTA, protease inhibitor), and protein concentration of the lysate was measured using bicinchronic acid assay. Twenty µg of protein was loaded on 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis, transferred to nitrocellulose membrane, blocked with 5% (w/v) non-fat milk solution in TBST with 0.1% (v/v) Tween 20 for 1 hour, and applied with primary antibodies. Used antibodies were goat anti-human SVCT-1 (1:200), SVCT-2 (1:200), and γ-tubulin (1:2,000) antibodies. After overnight incubation at 4℃, samples were incubated with horseradish peroxidase (HRP)–conjugated anti-goat IgG (1:10,000) or HRP-conjugated antimouse IgG (1:5,000) for 1 hour at room temperature (RT), and color reaction was performed using ECL detection kit (Amersham, GE Healthcare, Buckinghamshire, UK). All antibodies used were from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
T cells were attached on glass slides by incubating for 1 hour at RT, washed with phosphate buffered saline (PBS) for 3 times, and fixed with 4% paraformaldehyde solution for 20 minutes. After washing, T cells were treated with 10% normal donkey serum (Vector Laboratories, Burlingame, CA, USA) for 1 hour at RT, incubated with primary antibodies for 1 hour at RT, after then with donkey anti-goat Alexa 555 (1:500, Invitrogen) for 1 hour at RT. Primary antibodies used were goat anti-human SVCT-1, and anti-hunman SVCT-2 antibodies (both 1:100, Santa Cruz Biotechnology). Nuclear staining were carried out with DAPI solution. Slides were covered with mounting solution (Cat. No. S3025, DakoCytomation, Carpinteria, CA, USA) and observed under confocal microscope.
Vitamin C concentration was measured using a modified 2,4-dinitrophenylhydrazine (DNPH) method as previously described [18]. Briefly, T cells were lyzed by repeated freezing and thawing. Supernatants were obtained and mixed with equal volume of 10% metaphosphoric acid (Sigma), centrifuged, and supernatants were obtained again, into which 0.027 M cupric acid (Sigma), 0.68 M thiourea (Katayaka Chemical JIS, Osaka, Japan), and 0.1 M DNPH (Sigma) were sequentially added. The mixture was incubated in a 37℃ water bath for 3 hours to obtain red precipitates, which were melted by adding 12 M sulfuric acid. The absorbance at 520 nm was measured.
Forty-eight hours after activation, T cells were added with 1 µCi/well of [3H]-thymidine (American Radiolabeled Chemicals, St. Louis, MO, USA) for 16 hours and harvested, and the radioactivities were measured in a scintillation β-counter (MicroBeta, Trilux, PerkinElmer, Turku, Finland). All samples were quadripicated.
For apoptosis analysis, T cells were cultured in a 24-well plate in a CO2 incubator at 37℃, added with various concentrations of vitamin C up to 1 mM for 2 hours, and activated with PMA/ionomycin. After 24 hours, 1×106 cells were suspended in Annexin V binding buffer (BD Pharmingen, Franklin Lakes, NJ, USA) and incubated with Annexin V (BD Pharmingen) for 15 minutes. Propidium iodide (PI) (BD Pharmingen) was added just before flow cytometric analysis.
For the analysis of activation marker expression, human T cells in a fluorescence-activated cell sorting (FACS) tube (1×106/tube) were washed twice in cold PBS containing 0.05% bovine serum albumin (Amresco, Solon, OH, USA), incubated with anti-human CD69-FITC (BD Pharmingen) and anti-human CD25-FITC (BD Pharmingen) antibodies, 0.2 µg each on ice, and subjected to flow cytometric analysis.
Flow cytometric analysis was performed using FACS Calibur (BD Biosciences, San Diego, CA, USA).
Human T cells (5×105/well) were cultured in a 24-well plate
and activated with PMA/ionomycin. Vitamin C was added 2 hours before or 24 hours after activation at a concentration of 500 mM. Culture sup was obtained 36 hours after activation and enzyme-linked immunosorbent analysis (ELISA) for IL-2 was performed using IL-2 ELISA kit (Invitrogen) following the manufacturer's instruction.
In plasma, vitamin C is present dominantly as its reduced form, AA [27], which is taken up into cells via SVCTs. Many vital organs express SVCT1 and/or SVCT2 to use AA [2829]. However, it is not evidenced whether T cells express SVCTs. To figure out this point, we performed reverse transcription polymerase chain reaction (RT-PCR) and western blotting for these molecules in T cells. In this experiment, we used human hepatoma-derived cell line HepG2 which is known to express both SVCTs [30] as a reference to validate the data from T cells. RT-PCR revealed the expression of SVCT2, but not SVCT1 in human T cells (Fig. 1A). Furthermore, the expression increased after activation with PMA/ionomycin. Same results were obtained at the protein level, too (Fig. 1B). Again, when we immuno-stained T cells for SVCTs, signals for SVCT2, but not for SVCT1 were observed on the surface of naive T cells, becoming augmented 24 and 48 hours after activation (Fig. 1C).
To functionally confirm the presence of SVCT2 in human T cells, we activated T cells for 48 hours and added 0.5 mM vitamin C. Because vitamin C is known to immediately be converted to DHA in culture media [31] and thus transported into cells via GLUTs, we added 3 mM dithiothreitol (DTT) in culture media to prevent the conversion. Also, in another well, we used Na+-free buffer to block the action of SVCTs. Two hours after vitamin C addition, cells were harvested and lyzed, and intracellular vitamin C concentrations were measured as described in "Materials and Methods." With vitamin C addition, cellular level of it was substantially increased compared to those without vitamin C addition (Fig. 2, left two bars). When DTT was added in culture media, vitamin C uptake was decreased (Fig. 2, middle bar) compared to those with vitamin C only, but still increased compared to those without vitamin C, reflecting the uptake via SVCTs, especially SVCT2 in this case. When the function of SVCTs was blocked by sodium-depletion (Fig. 2, second-to-the-right bar), it still showed substantial uptake of vitamin C, implicating the massive conversion of AA to DHA and absorption of it via GLUTs. Lastly, when transports via SVCTs and GLUTs were all blocked by sodium depletion and DTT addition (Fig. 2, right-most bar), cellular vitamin C level was similar to that without vitamin C.
These results in concert indicate the presence of functional SVCT2 in human T cells.
As vitamin C supplementation in human has been reported to augment proliferating activity of human T cells [152132], we treated the cells with increasing concentrations of vitamin C, from a physiological concentration (62.5 µM [32]) to mega-dose (1 mM) for 2 hours, activated them with PMA/ionomycin or anti-CD3/CD28 antibodies for 48 hours, and performed [3H]-thymidine uptake assay. As a result, T-cell proliferation did not show any discernable changes with low concentrations of vitamin C, but was inhibited with higher concentrations, that is, with 500 and 1,000 mM, regardless of the activation mode (Fig. 3A). This was against what was expected.
We wondered whether the inhibitory effects of high concentration vitamin C were exerted at the beginning of activation, and/or during activation. Thus, we repeated same experiments with vitamin C being added 24 hours after activation. In this time, vitamin C did not affected T-cell proliferation at all, regardless of the concentrations added (Fig. 3B).
To further analyze the time when treated vitamin C exerted toxic effects, we added 1 mM vitamin C into 96-well cultures (1×105 cells/well) at 3, 6, 12, and 24 hours after activation with PMA/ionomycin, and [3H]-thymidine uptake analysis was performed. A partial toxic effect was observed with 3-hour treated group (Fig. 4). Thus, at least in vitro, high concentration of vitamin C was harmful for T-cell proliferation when it was given before activation and at least within 3 hours after activation.
To elucidate whether the decreased thymidine uptake by T cells in the presence of high concentration vitamin C was due to cell death, T cells were cultured with the addition of 1 mM vitamin C 2 hours before or 24 hours after activation using PMA/ionomycin. Cells were harvested 48 hours after activation and stained with Annexin V and PI, then subjected to flow cytometric analysis. About 30% of T cells without vitamin C showed apoptotic figures (Fig. 5, left panel), while those treated with vitamin C before activation showed nearly 60% frequency of apoptosis (Fig. 5, middle panel). Again, T cells treated 24 hours after activation showed similar features to that of control group (Fig. 5, right panel), consistent with results shown in Figs. 3 and 4.
Because high concentration of vitamin C induced apoptosis of T cells when it was given before activation, we raised a question whether the same condition affected the activation of survived T cells. Cells were activated with PMA/ionomycin and maintained for 24 hours, during which 1 mM vitamin C was added at various times before and after activation. After then, cells were stained for the surface expression of CD69 or CD25, the early activation markers of T cells, and subjected to flow cytometric analysis. As results, the expression frequency of CD69 was not affected by vitamin C treatment regardless of the application time. However, a decrease of the mean fluorescence intensity (MFI) value was observed in the pretreated group (–2 hours in Fig. 6). On the contrary, there was a marked decrease of CD25 expression frequency, almost 70% compared to the control group. A lowered MFI value also was observed in this group.
Another hallmark of T-cell activation is the secretion of IL-2, which is a trophic factor for T cells and is important for cell cycle progress and clonal expansion of T cells [33]. Thus, we evaluated the IL-2–secreting capacity of vitamin C–treated T cells. Cells (5×105/well) were plated and activated with PMA/ionomycin. Vitamin C was added at 500 µM 2 hours before or 24 hours after activation. Supernatans were obtained 36 hours after activation and IL-2 ELISA was performed. As results, pre-treatment of vitamin C substantially reduced IL-2 secretion, while after-treatment reduced it only slightly without statistical significance (P=0.11).
This study was performed to analyze the mode of vitamin C uptake in human T cells and to evaluate direct effects of vitamin C on these cells.
As previously described, vitamin C is taken up as DHA through GLUTs or as AA through SVCTs. That means DHA can be taken up by every kinds of cells. However, it is not the case for AA, because the specific transporters SVCT1 and 2 are not present ubiquitously in the body but confined to some organs such as small intestine, liver, kidney, adrenal gland, brain, retina, and others [34]. The presence of SVCTs in human T cells remained uncertain until we observed the expression of functional SVCT2, but not SVCT1 in this experiment (Figs. 1, 2). In fact, the presence of SVCT1 and/or SVCT2 in human T cells could be predicted based on previous reports. Peripheral blood lymphocytes, which are largely comprised of T cells [35], accumulate up to 80-fold concentration of vitamin C compared to that in serum [22], and majority of vitamin C in the serum is present as its reduced form, AA [7], which together suggest the presence of a mechanism in T cells that actively takes up AA. Another report also support this prediction that lymphocytes transport vitamin C effectively and intracellular vitamin C seems to be saturated even under low dietary intake [36].
What is interesting is that the expression of SVCT2 in T cells increased after activation (Fig. 1). This suggests a certain beneficial role of vitamin C during T-cell activation. However, the results were against our expectation. That is, in vitro vitamin C did not augment the proliferation of T cells (Fig. 3), contrary to what was observed in T cells from vitamin C– supplemented men [1521]. Rather, at higher concentrations, pre-treated vitamin C decreased proliferation, expression of activation markers, and IL-2 secretion. In addition. it increased the frequency of apoptotic cells following activation (Figs. 3, 4, 5). These results indicate an inhibitory role of preteated high concentration vitamin C. Similar results have been reported previously in mouse T cells [18], in which 0.5 mM vitamin C in culture media decreased proliferation and cytokine secretion (tumor necrosis factor α, interferon γ, and IL-4), and in human peripheral T cells and lymphocytes [3738], in which the presence of vitamin C decreased viability and IL-2 secretion. However, there is no explanation for these inhibitory effects.
With activation, T cells begin to accumulate intracellular ROS within minutes [39], and the level peaks 1–2 hours after activation and then declines during the following 6 hours [40]. These ROS are mainly derived from mitochondria [41] and considered to be related to T-cell activation [2441]. Many investigators suggested the role of ROS by inhibiting T-cell activation using several kinds of anti-oxidants. Ferricyanide, iron chelators, or free radical scavenger (butylated hydroyanisole), when applied at the time of T-cell activation, all suppressed cell proliferation and CD25 expression [42]. Again, lipo-oxygenase inhibitors, hydroxyl radical scavengers, or oxygen radical scavengers also suppressed T-cell proliferation and IL-2 secretion [43]. More specifically, blocking ROS formation using complex I inhibitors such as rotenone, or metformin, resulted in down-regulation of of IL-2, IL-4, and FasL [4445]. Because vitamin C also is an antioxidant, we could raise an assumption that the toxic effect of high concentration vitamin C, suppression of proliferation, IL-2 secretion, and CD25 expression, could be exerted by reducing intracellular ROS level. In fact, decrease of ROS level in human T cells has been reported by addition of 0.5 µM vitamin C 30 minutes before activation in vitro [39].
However, the suppression of proliferation occurred at a too high concentration, 0.5 mM (Fig. 3). At this concentration, intracellular ROS was complete eradicated [39]. Thus, this suppression could be a result of pro-oxidant effects of vitamin C rather than anti-oxidant effects, because vitamin C acts not only as an antioxidant but also as a pro-oxidant, particularly at high concentrations [46].
Apoptotic profile also seems to deny anti-oxidant effects of vitamin C in this experiment. ROS is known to promote activation-induced cell death (AICD) of T cells by elevating FasL expression and down-regulation of Bcl-2 [2347]. These effects are via activation of nuclear factor of activated T cells and NF-κB, respectively. Thus, antioxidants suppressed AICD [4849]. Our results showed increased frequency of apoptosis with vitamin C pre-treatment (Figs. 2, 3), contrary to the above-mentioned reports.
Based above considerations, we assume that pre-treated vitamin C did not exert anti-oxidant effects during T-cell activation at given concentrations. Instead, it seems to act as a pro-oxidant at high concentrations.
The question is that why vitamin C, even at lower concentrations, did not exert any effects at all, even though it is certainly an "anti-oxidant" and vividly taken up by those cells (Fig. 2). One possibility is that there are various kinds of antioxidant systems already set. Those include enzymes such as syperoxide dismutase, catalase, glutathione peroxidase and so on, and non-enzymatic small molecules such as pyruvate, α-ketoglutarate, and oxaloacetate [50]. Thus, exogenous vitamin C might have no chance to affect mitochondrial ROS that is essential for T-cell activation [41]. Or, more probably, ascorbate did not interfere ROS needed for T-cell activation. As an anti-oxidant, ascorbate reacts with single oxygen to clear it up to yield superoxide, which immediately converts to H2O2 [51]. These superoxide and hydrogen peroxide are regarded as predominant mitochondrial ROS acting in T-cell activation [5253]. Thus, absorbed vitamin C could elevate the ROS signal level, instead of lowering it. For now, it is uncertain what the exact mechanism via which vitamin C exerts its toxic effects is. To be noted is that the toxic effects did appear only when it was given before or immediately after activation (Figs. 3, 4, 6, 7). Further studies are needed and would be better to be focused at the initial stage of T-cell activation.
In conclusion, we found the expression of SVCT2 in human T cells, which level increased with activation. In addition, we also found that vitamin C did not act as anti-oxidant but exerted toxic effects with high concentrations during the initial phase of human T-cell activation. Whether these phenomena are reflected in vivo is to be determined.
Figures and Tables
 | Fig. 1SVCT-2 expression in human T cells. (A) Total RNA was extracted from human T cells and cDNA was synthesized. The expression of SVCT-1 and -2 was assessed using reverse transcription polymerase chain reaction. (B) T cells were lysed and subjected to western blotting for the expression of SVCT-2. HepG2 (hepatoma cell line) cells were used as positive control of SVCT-1 and -2. (C) Human T cells were collected 24 hours or 48 hours after activation with phorbol-12-myristate-13-acetate/ionomycin, attached on a slide, and briefly fixed with formalin. The expression of SVCT-2 was assessed by immunofluorescence staining. The staining was observed under a confocal microscope. |
 | Fig. 2Vitamin C uptake by human T cells. Cells were activated with phorbol-12-myristate-13-acetate/ionomycin for 48 hours, re-allocated in a various culture condition (4×106 cells/group), and added with vitamin C at a concentration of 0.5 mM. After 2 hours, cells were harvested and vitamin C concentration was measured using DNPH method. The experiment was repeated 3 times with triplicated samples per each experiment, and a representative one is presented. No VC, not treated with vitamin C; VC, treated with vitamin C; DTT+VC, treated with vitamin C in the presence of dithiothreitol (DTT); Na+ free buffer+VC, cells stayed in Na+ free buffer with vitamin C; Na+ free buffer+ DTT+VC, cells stayed in Na+ free buffer with vitamin C and DTT in it. **P<0.01. |
 | Fig. 3The effects of vitamin C on proliferation of human T cells. Cells (1×105/well) were plated in flat bottom 96-well and activated with phorbol-12-myristate-13-acetate (PMA)/ionomycin or anti-CD3 and anti-CD28 antibodies. Vitamin C was treated 2 hours before (A) or 24 hours after activation (B) with various concentrations of vitamin C as indicated. [3H]-thymidine was added at 48 hours, cultured additional 16 hours, and counter per minute (CPM) values were measured. The experiment was repeated three times and a representative one is shown. All the samples were quadriplicated. **P<0.01. |
 | Fig. 4Human T cells (1×105 cells/well) were cultured in a 96-well plate and activated with phorbol-12-myristate-13-acetate (PMA)/ionomycin. Cells were treated with 0.5 mM vitamin C 3, 6, 12, or 24 hours after activation, added with [3H]-thymidine at 48 hours, cultured for additional 16 hours, and counter per minute (CPM) values were measured. All the samples were quadriplicated. The experiment was repeated three times and a representative one is shown. Control group was cultured without vitamin C treatment. **P<0.01. |
 | Fig. 5The effects of vitamin C on human T cell apoptosis. Cells were activated with phorbol-12-myristate-13-acetate/ionomycin, added with 0.5 mM vitamin C 2 hours before or 24 hours after activation, stained with Annexin V and propidium iodide (PI), and subjected to flow cytometric analysis. The experiment was repeated three times and a representative one is shown. Control group was cultured without vitamin C treatment. |
 | Fig. 6Expression of CD69 and CD25 under vitamin C treatment at various times before and after activation. Human T cells (5×105 cells/well) were cultured for 2 hours in a 24-well plate with 1 mM vitamin C before phorbol-12-myristate-13-acetate/ionomycin activation and 3, 6, and 12 hours after activation Twenty-four hours after activation, cells were harvested, stained with appropriate antibodies, and were subjected to flow cytometric analysis. The experiment was repeated three times and a representative one is shown. Numbers are in %. SSC, side-scattered light; FSC, forward-scattered light. |
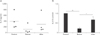 | Fig. 7Effects of high concentration vitamin C on interleukin 2 (IL-2) secretion in human T cells. Cells from 4 individuals were cultured in a 24-well plate and activated with phorbol-12-myristate-13-acetate/ionomycin. Vitamin C was added to the culture media 2 hours before or 24 hours after activation, or not added (control). Culture supernatants were obtained at 36 hours and IL-2 amount were analysed by enzymelinked immunosorbent analysis. Data are presented as absolute concentrations (A) or normalized values (B) to the control data. Samples were quadriplicated. *P<0.05, **P<0.01. |
Acknowledgements
This work was supported by the Education and Research Encouragement Fund of Seoul National University Hospital (2015).
References
1. Frei B, England L, Ames BN. Ascorbate is an outstanding antioxidant in human blood plasma. Proc Natl Acad Sci U S A. 1989; 86:6377–6381.
2. Rumsey SC, Kwon O, Xu GW, Burant CF, Simpson I, Levine M. Glucose transporter isoforms GLUT1 and GLUT3 transport dehydroascorbic acid. J Biol Chem. 1997; 272:18982–18989.
3. Vera JC, Rivas CI, Fischbarg J, Golde DW. Mammalian facilitative hexose transporters mediate the transport of dehydroascorbic acid. Nature. 1993; 364:79–82.
4. Faaland CA, Race JE, Ricken G, Warner FJ, Williams WJ, Holtzman EJ. Molecular characterization of two novel transporters from human and mouse kidney and from LLC-PK1 cells reveals a novel conserved family that is homologous to bacterial and Aspergillus nucleobase transporters. Biochim Biophys Acta. 1998; 1442:353–360.
5. Daruwala R, Song J, Koh WS, Rumsey SC, Levine M. Cloning and functional characterization of the human sodium-dependent vitamin C transporters hSVCT1 and hSVCT2. FEBS Lett. 1999; 460:480–484.
6. Rajan DP, Huang W, Dutta B, Devoe LD, Leibach FH, Ganapathy V, Prasad PD. Human placental sodium-dependent vitamin C transporter (SVCT2): molecular cloning and transport function. Biochem Biophys Res Commun. 1999; 262:762–768.
7. Tsukaguchi H, Tokui T, Mackenzie B, Berger UV, Chen XZ, Wang Y, Brubaker RF, Hediger MA. A family of mammalian Na+-dependent L-ascorbic acid transporters. Nature. 1999; 399:70–75.
8. Nishikimi M, Fukuyama R, Minoshima S, Shimizu N, Yagi K. Cloning and chromosomal mapping of the human nonfunctional gene for L-gulono-gamma-lactone oxidase, the enzyme for L-ascorbic acid biosynthesis missing in man. J Biol Chem. 1994; 269:13685–13688.
9. National Institutes of Health. Vitamin C [Internet]. Bethesda, MD: National Institutes of Health;2016. cited 2016 Mar 1. Available from: https://ods.od.nih.gov/factsheets/VitaminCConsumer/.
10. Johnston CS. Biomarkers for establishing a tolerable upper intake level for vitamin C. Nutr Rev. 1999; 57:71–77.
11. Noh K, Lim H, Moon SK, Kang JS, Lee WJ, Lee D, Hwang YI. Mega-dose vitamin C modulates T cell functions in Balb/c mice only when administered during T cell activation. Immunol Lett. 2005; 98:63–72.
12. Prinz W, Bortz R, Bregin B, Hersch M. The effect of ascorbic acid supplementation on some parameters of the human immunological defence system. Int J Vitam Nutr Res. 1977; 47:248–257.
13. Feigen GA, Smith BH, Dix CE, Flynn CJ, Peterson NS, Rosenberg LT, Pavlović S, Leibovitz B. Enhancement of antibody production and protection against systemic anaphylaxis by large doses of vitamin C. Res Commun Chem Pathol Pharmacol. 1982; 38:313–333.
14. Anderson R, Dittrich OC. Effects of ascorbate on leucocytes: Part IV. Increased neutrophil function and clinical improvement after oral ascorbate in 2 patients with chronic granulomatous disease. S Afr Med J. 1979; 56:476–480.
15. Jeong YJ, Hong SW, Kim JH, Jin DH, Kang JS, Lee WJ, Hwang YI. Vitamin C-treated murine bone marrow-derived dendritic cells preferentially drive naive T cells into Th1 cells by increased IL-12 secretions. Cell Immunol. 2011; 266:192–199.
16. Kennes B, Dumont I, Brohee D, Hubert C, Neve P. Effect of vitamin C supplements on cell-mediated immunity in old people. Gerontology. 1983; 29:305–310.
17. Cetinkale O, Senel O, Bulan R. The effect of antioxidant therapy on cell-mediated immunity following burn injury in an animal model. Burns. 1999; 25:113–118.
18. Maeng HG, Lim H, Jeong YJ, Woo A, Kang JS, Lee WJ, Hwang YI. Vitamin C enters mouse T cells as dehydroascorbic acid in vitro and does not recapitulate in vivo vitamin C effects. Immunobiology. 2009; 214:311–320.
19. Woo A, Kim JH, Jeong YJ, Maeng HG, Lee YT, Kang JS, Lee WJ, Hwang YI. Vitamin C acts indirectly to modulate isotype switching in mouse B cells. Anat Cell Biol. 2010; 43:25–35.
20. Jeong YJ, Kim JH, Hong JM, Kang JS, Kim HR, Lee WJ, Hwang YI. Vitamin C treatment of mouse bone marrow-derived dendritic cells enhanced CD8(+) memory T cell production capacity of these cells in vivo. Immunobiology. 2014; 219:554–564.
21. Anderson R, Oosthuizen R, Maritz R, Theron A, Van Rensburg AJ. The effects of increasing weekly doses of ascorbate on certain cellular and humoral immune functions in normal volunteers. Am J Clin Nutr. 1980; 33:71–76.
22. VanderJagt DJ, Garry PJ, Bhagavan HN. Ascorbate and dehydroascorbate: distribution in mononuclear cells of healthy elderly people. Am J Clin Nutr. 1989; 49:511–516.
23. Williams MS, Kwon J. T cell receptor stimulation, reactive oxygen species, and cell signaling. Free Radic Biol Med. 2004; 37:1144–1151.
24. Belikov AV, Schraven B, Simeoni L. T cells and reactive oxygen species. J Biomed Sci. 2015; 22:85.
25. García Mde L, Salazar K, Millán C, Rodríguez F, Montecinos H, Caprile T, Silva C, Cortes C, Reinicke K, Vera JC, Aguayo LG, Olate J, Molina B, Nualart F. Sodium vitamin C cotransporter SVCT2 is expressed in hypothalamic glial cells. Glia. 2005; 50:32–47.
26. Seno T, Inoue N, Matsui K, Ejiri J, Hirata K, Kawashima S, Yokoyama M. Functional expression of sodium-dependent vitamin C transporter 2 in human endothelial cells. J Vasc Res. 2004; 41:345–351.
27. Nualart FJ, Rivas CI, Montecinos VP, Godoy AS, Guaiquil VH, Golde DW, Vera JC. Recycling of vitamin C by a bystander effect. J Biol Chem. 2003; 278:10128–10133.
28. Wang Y, Mackenzie B, Tsukaguchi H, Weremowicz S, Morton CC, Hediger MA. Human vitamin C (L-ascorbic acid) transporter SVCT1. Biochem Biophys Res Commun. 2000; 267:488–494.
29. Takanaga H, Mackenzie B, Hediger MA. Sodium-dependent ascorbic acid transporter family SLC23. Pflugers Arch. 2004; 447:677–682.
30. Reidling JC, Subramanian VS, Dahhan T, Sadat M, Said HM. Mechanisms and regulation of vitamin C uptake: studies of the hSVCT systems in human liver epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2008; 295:G1217–G1227.
31. Clement MV, Ramalingam J, Long LH, Halliwell B. The in vitro cytotoxicity of ascorbate depends on the culture medium used to perform the assay and involves hydrogen peroxide. Antioxid Redox Signal. 2001; 3:157–163.
32. Berger J, Shepard D, Morrow F, Taylor A. Relationship between dietary intake and tissue levels of reduced and total vitamin C in the nonscorbutic guinea pig. J Nutr. 1989; 119:734–740.
33. Malek TR. The biology of interleukin-2. Annu Rev Immunol. 2008; 26:453–479.
34. Savini I, Rossi A, Pierro C, Avigliano L, Catani MV. SVCT1 and SVCT2: key proteins for vitamin C uptake. Amino Acids. 2008; 34:347–355.
35. Amatya R, Vajpayee M, Kaushik S, Kanswal S, Pandey RM, Seth P. Lymphocyte immunophenotype reference ranges in healthy Indian adults: implications for management of HIV/AIDS in India. Clin Immunol. 2004; 112:290–295.
36. Jacob RA, Pianalto FS, Agee RE. Cellular ascorbate depletion in healthy men. J Nutr. 1992; 122:1111–1118.
37. Eylar E, Báez I, Navas J, Mercado C. Sustained levels of ascorbic acid are toxic and immunosuppressive for human T cells. P R Health Sci J. 1996; 15:21–26.
38. Härtel C, Strunk T, Bucsky P, Schultz C. Effects of vitamin C on intracytoplasmic cytokine production in human whole blood monocytes and lymphocytes. Cytokine. 2004; 27:101–106.
39. Tatla S, Woodhead V, Foreman JC, Chain BM. The role of reactive oxygen species in triggering proliferation and IL-2 secretion in T cells. Free Radic Biol Med. 1999; 26:14–24.
40. Kamiński MM, Röth D, Sass S, Sauer SW, Krammer PH, Gülow K. Manganese superoxide dismutase: a regulator of T cell activation-induced oxidative signaling and cell death. Biochim Biophys Acta. 2012; 1823:1041–1052.
41. Murphy MP, Siegel RM. Mitochondrial ROS fire up T cell activation. Immunity. 2013; 38:201–202.
42. Chaudhri G, Hunt NH, Clark IA, Ceredig R. Antioxidants inhibit proliferation and cell surface expression of receptors for interleukin-2 and transferrin in T lymphocytes stimulated with phorbol myristate acetate and ionomycin. Cell Immunol. 1988; 115:204–213.
43. Dornand J, Gerber M. Inhibition of murine T-cell responses by anti-oxidants: the targets of lipo-oxygenase pathway inhibitors. Immunology. 1989; 68:384–391.
44. Kaminski MM, Sauer SW, Klemke CD, Süss D, Okun JG, Krammer PH, Gülow K. Mitochondrial reactive oxygen species control T cell activation by regulating IL-2 and IL-4 expression: mechanism of ciprofloxacin-mediated immunosuppression. J Immunol. 2010; 184:4827–4841.
45. Owen MR, Doran E, Halestrap AP. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J. 2000; 348 Pt 3:607–614.
46. Podmore ID, Griffiths HR, Herbert KE, Mistry N, Mistry P, Lunec J. Vitamin C exhibits pro-oxidant properties. Nature. 1998; 392:559.
47. Hildeman DA. Regulation of T-cell apoptosis by reactive oxygen species. Free Radic Biol Med. 2004; 36:1496–1504.
48. Bauer MK, Vogt M, Los M, Siegel J, Wesselborg S, Schulze-Osthoff K. Role of reactive oxygen intermediates in activationinduced CD95 (APO-1/Fas) ligand expression. J Biol Chem. 1998; 273:8048–8055.
49. Sandstrom PA, Mannie MD, Buttke TM. Inhibition of activationinduced death in T cell hybridomas by thiol antioxidants: oxidative stress as a mediator of apoptosis. J Leukoc Biol. 1994; 55:221–226.
50. Nathan C, Cunningham-Bussel A. Beyond oxidative stress: an immunologist's guide to reactive oxygen species. Nat Rev Immunol. 2013; 13:349–361.
51. Chou PT, Khan AU. L-ascorbic acid quenching of singlet delta molecular oxygen in aqueous media: generalized antioxidant property of vitamin C. Biochem Biophys Res Commun. 1983; 115:932–937.
52. Kamiński MM, Röth D, Krammer PH, Gülow K. Mitochondria as oxidative signaling organelles in T-cell activation: physiological role and pathological implications. Arch Immunol Ther Exp (Warsz). 2013; 61:367–384.
53. Sena LA, Li S, Jairaman A, Prakriya M, Ezponda T, Hildeman DA, Wang CR, Schumacker PT, Licht JD, Perlman H, Bryce PJ, Chandel NS. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity. 2013; 38:225–236.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download