Abstract
Figures and Tables
Fig. 1
Elastic fiber-rich fascia attaching to the posterior aspect of the joint capsule. Sagittal sections of a specimen from an 81-year-old man. All panels show near sections. (A) The left-hand side of the panel corresponds to the anterior side of the body (elastica Masson staining). The joint cartilage is larger on the arytenoid side than on the cricoid side. Surface roughness is evident (A). The posterior cricoarytenoid muscle (PCAM) attaches to the joint, and a covering fascia (arrowheads) of the muscle contains abundant elastic fibers (black color). Synovial folds (SF) are triangular in shape on the posterior side of the joint, whereas they are beltlike on the anterior side. Panels (B) (immunohistochemistry for elastin), corresponding to the squares in panel (A), display strong elastin expression in the posterior fascial structure (arrowheads) supporting the joint. SF in panel (C) (immunohistochemistry for elastin) are shown in panels (D–I) using immunohistochemistry. (D) Multiple blood capillaries in the anterior fold (immunohistochemistry for factor VIII). (E–I) A few lymphocytes and macrophages (arrows) in the anterior fold ( immunohistochemistr y for CD79a, CD68, IgM, CD3, and CD8, respectively). AC, arytenoid cartilage; CC, cricoid cartilage. Scale bars=1 mm (A–D), 0.1 mm (E–I).
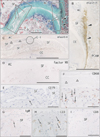
Fig. 2
Few elastic fibers in a fascia attached to the lateral aspect of the joint capsule. Sagittal sections of a specimen from an 89-year-old man. All panels show near sections. (A) The left-hand side of the panel corresponds to the anterior side of the body (elastica Masson staining). The lateral cricoarytenoid muscle (LCAM) is closely located to the joint. Part of the cricoid cartilage (asterisk) has been damaged during the histological procedure. Two long recesses of the joint cavity are seen on the anterior side. Panel (B) (immunohistochemistry for elastin), corresponding to the square in panel (A), displays elastin expression in a fascia (arrowheads) of the posterior cricoarytenoid muscle (PCAM). (C, D) Macrophages (arrows) in the synovial folds: they are rich in the fatty tissue on the anterior side of the joint (C), whereas they are sparse on a posterior fold (D) (immunohistochemistry for CD68). AC, arytenoid cartilage; CC, cricoid cartilage; SF, synovial fold. Scale bars=1 mm (A, B), 0.1 mm (C, D).
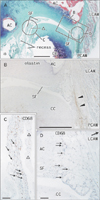
Fig. 3
No or few supportive structures along the joint capsule. Sagittal sections of a specimen from a 97-year-old woman. All panels show near sections. (A) The left-hand side of the panel corresponds to the anterior side of the body (H&E staining). The lateral cricoarytenoid muscle (LCAM) is located near the joint. Synovial folds (SF) are triangular in shape on the posterior side of the joint, whereas they are tongue-like on the anterior side. (B, F) A few macrophages on the SF (immunohistochemistry for CD68). Panels (C), (D) and (E) (immunohistochemistry for factor VIII, IgM, and CD8, respectively) exhibit few blood capillaries (C) and lymphocytes (D, E) in the posterior triangular fold. Arrows indicate macrophage. AC, arytenoid cartilage; CC, cricoid cartilage. Scale bars=1 mm (A), 0.1 mm (B–F).
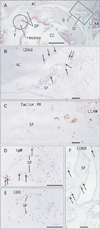
Fig. 4
Schematic drawings of the carpometacarpal joint of the thumb and cricoarytenoid joint. Asterisk is the carpometacarpal joint of the thumb (A). Stars are the cricoarytenoid joint (B). The cricoarytenoid joint is similar to a saddle joint, such as the the carpometacarpal joint of the thumb.
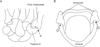
Table 1
Morphology of the CA joint and correlation with CT joint degeneration
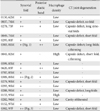
| Synovial fold | Posterior elastic band | Macrophage density | CT joint degeneration | |
|---|---|---|---|---|
| 0150, 62M | + | - | Low | - |
| 0037, 74M | + | + | Low | Capsule defect, no fold |
| 0278, 75F | ++ | + | Low | Capsule defect, long syno- |
| 0049, 76M | + | - | Low | Capsule defect, short fold |
| 0295, 80F | + | + | Low | - |
| 0303, 81M | + (Fig. 1) | ++ | Low | Capsule defect; long folds; |
| 0041, 82M | + | - | High | Capsule defect, short fold, c.thinning |
| 0599, 83M | + | + | Low | - |
| 0620, 83F | + | ++ | Low | - |
| 0707, 85M | + | - | Low | - |
| 0309, 89M | ++ (Fig. 2) | + | Low | - |
| 0279, 90M | + | - | Low | Capsule defect, short fold |
| 0293, 90M | + | - | Low | - |
| 0288, 91M | + | - | Low | Capsule defect, long folds |
| 0153, 93M | ++ | - | High | - |
| 0282, 94M | + | + | Low | Cavity obliterated |
| 0152, 97M | + | + | Low | - |
| 0302, 97F | ++ (Fig. 3) | - | Low | Capsule defect, short fold |
CA joint, cricoarytenoid joint; CT joint, cricothyroid joint. Synovial fold: +, long folds (over 1 mm) present in the lateral aspect of the joint; ++, present in both the lateral and medial aspects. Posterior elastic band: +, a thickened fascia of the posterior cricoarytenoid muscle attached to, or extending near, the lateral aspect of the CA joint capsule; ++, a long band connecting the arytenoid to the cricoid cartilage. Macrophage density: low or high, fewer or more than 10 positive cells per 100 µm2 along the joint cavity upon immunohistochemistry for CD68. CT joint degeneration had been examined in a half of the present 18 cadavers (Serikawa et al. [1]): the capsule defect caused exposure of the ligament elastic fiber components to the joint cavity; c.thinning, cartilage thinning; cavity obliterated, the joint cavity was filled with elastic fiber-rich fibrous tissue with few macrophages.
Table 2
Comparison of age-related degenerative morphology between the cricoarytenoid and cricothyroid joints

Observations of the cricothyroid joint are based on Serikawa et al. [1].




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download