Abstract
Superficial temporal artery (STA) based pedicled fascial flap plays a pivotal role in ear reconstruction for microtia patients. There is paucity of literature on the anatomy of the STA in microtia patients. The present study aimed to describe any possible anatomical variations seen in the STA of patients afflicted with microtia. Pre-operative carotid computer tomographic angiography images of patients under the microtia database of Plastic and Reconstructive Surgery Unit at a tertiary medical centre were selected and 3-dimensionally reconstructed. Measurements were made on the 3D reconstructed computed tomographic angiography images of the STA on both the sides of the microtic ear and the non-microtic ear to assess its various anatomical parameters. We managed to obtain a total of 39 computed tomographic angiography images of STAs for analysis. There was a significant difference in the number of main branches of STA between the two groups (P=0.006). The proportion of ears with 2 main branches was higher in the non-microtia group (89.5%) compared to the microtia group (45.0%). A significant difference was found in the STA diameter between the two groups (P=0.012). The mean diameter of STA in the non-microtia group was larger by 0.4 mm. Furthermore, the median angle of STA was larger on the side of the non-microtic ears compared to that of microtic ears by 24.5°, with a P-value of 0.011. The results of the study may be of clinical importance while planning and performing ear reconstructive surgeries using STA based pedicled fascial flaps.
The anatomy of the superficial temporal artery (STA) and its branches has been extensively studied before. It is one of the terminal branches that arises from the external carotid artery, running up behind the temporo-mandibular joint, emerging from the tissue of the parotid gland, deep to the neck of the mandible, and crosses superficially over the posterior root of the zygomatic process of the temporal bone. It gives off the middle temporal artery, and traverses along the pre-auricular area before dividing at or above the zygomatic arch into its two terminal branches, i.e., anterior/frontal branch, and the posterior/parietal branch [12]. STA and branches of the posterior auricular artery are the primary source of blood supply to the external ear.
Previous research studies involving STA looked into the diameter, branching pattern, as well as relation to the pericranial structures, e.g., the point of origin of the main branches of the STA in relation to the zygomatic arch—whether it was above, on, or below the arch [345]. All these studies were performed mainly by analysis of cadaveric dissection samples on the normal populations. They all demonstrated the preponderance of level of bifurcation of the STA above the zygomatic arch. The observed inter-studies variations were believed to be attributed to ethnological differences. Meanwhile, Marano et al. [4] had described up to 10 different groups of STA variations in the normal population based on its diameters, branching patterns and levels of branching in relation to the zygomatic arch. STA anomaly in hemifacial microsomia patients with microtia were briefly mentioned by Yamada et al. [6]. They observed that only the frontal branch of the STA appears to be the main trunk, with virtually, no posterior branches. They also observed that the temporal muscle and the fascia were underdeveloped in this group. This could point to the possibility that variations do exist in STA anatomy and its distribution in the microtia population.
Microtia is the most commonly occurring congenital malformation of the external ear, with a prevalence of approximately 3 per 10,000 live birth. Occurrence has been reported to be 1 in 4,000 in the Japanese population and as high as 1 in 900 to 1 in 1,200 in the Navajo population of southwestern United States [78]. It can be an isolated deformity, which is commonly the case, or it can sometimes be part of a spectrum of other deformities, such as hemifacial microsomia and Goldenhar syndrome. The etiology of microtia has not been completely well understood, to date. Nevertheless, technical advances in the reconstruction of the microtic ear have brought about dramatically improved outcomes in the hands of experienced ear reconstructive surgeons. Many of the reconstruction techniques involved manipulation of pedicled temporoparietal fascial flaps based on the STA.
STA plays a pivotal role in microtia auricular reconstruction. The two most popular techniques in autogenous reconstruction of the microtic ear are those described by Brent [9] and Nagata [10]. In his popular four-stage reconstruction technique, Brent [9] first refined the concept previously pioneered by Tanzer [11]. Nagata [10] later analyzed the results and designed a two-stage technique, in which usage of a temporoparietal flap supplied by STA is a notable feature of the second stage. The well-known variability of the STA and its branches makes it important to ascertain the STA variations in microtia patients, if any, prior to surgery to minimized any potential post-operative complications [61213].
In an extant search of literature, we found that none of the published papers had explored the anatomical distributions of the STA in the microtia population. Hence, we embarked on this pilot study, using 3D computed tomographic angiography (CTA) imaging of STA, to determine its anatomical variants in patients with microtia. This information may be useful for academic purpose while providing important background knowledge on any STA variation and its association with microtia patients. The results of the present study may also be potentially helpful when applied to operative planning, e.g., in multi-staged auricular reconstructions involving the use of pedicled fascial flaps for microtia patients since STA supplies the scalp and face and it is also used for temporoparietal, parieto-occipital and forehead flaps in reconstructive surgery.
All microtia patients under the care of Plastic and Reconstructive Surgery Department, Universiti Kebangsaan Malaysia Medical Centre (UKMMC) between 2011 to 2015, were considered for the study. Prior ethical approval and consent was obtained for conducting the study. All the patients had undergone preoperative computed tomography (CT) angiography assessment of the head and neck vessels as per standard management workflow. The CTA images were obtained using a Siemens SOMATOM Sensation 64 slice eco CT system (Forchheim, Germany). A total of 100 ml of Iomeron 350 mg/ml (Patheon Italia [Bracco] S.p.A Corp., Ferentino, Italy) was used as pre-scan contrast, injected via cephalic vein at 5 ml/sec, and the scan was commenced after 15 seconds. The image data were stored in the standard DICOM format. Subjects with both congenital (true microtia) and acquired disease (those who sustained accidental/traumatic isolated ear tissue loss later in life) were included in the study. These patients had either unilateral or bilateral ear involvement.
Those excluded from the study were the ones with history of reconstructive surgery involving STA-based pedicled fascial flap on the microtic ear prior to CT angiography. Patients with history of deforming trauma to the peri-auricular region other than that only involving the ear/part of the ear were also excluded from the study. Based on the criteria fulfilled, a final sample size of 39 ears was obtained.
The CTA images were 3D reconstructed using Siemens syngo InSpace 3D software (Fig. 1A). Measurements were made on the 3D reconstructed images to assess, on both the sides of the microtic ear and the non-microtic ear, the (1) diameter of STA and each of its main branches, (2) number of main branches of STA, (3) level and distance of STA branching point in relation to the ipsilateral zygomatic arch, and (4) angle of STA and its main branches in relation to a plane drawn parallel to the upper border of the ipsilateral zygomatic arch (Fig. 1B–D). At least three measurements were taken for each of the above numerical parameters and the mean value was obtained for each.
The collected data were then tabulated and analysed using IBM SPSS Statistics version 20.0 (IBM Corp., Armonk, NY, USA). Baseline characteristics were summarized using appropriate descriptive statistics. Fisher exact test was used to analyze the association between two categorical variables. Independent t-test and Mann-Whitney test were used to analyze the association between categorical and numerical variables. The anatomy of the left and right ears was assumed to be independent. Normality of all numerical variables was assessed using Shapiro-Wilk test. All hypotheses tests were based on two-sided tests and P-values of < 0.050 were considered statistically significant.
The mean age was 20.4 years, with standard deviation of 9.1 years. Two third of the microtia patients were aged between 10 to 29 years. The male to female ration of microtia patients was 1.6:1 (13 males and 7 females). A total of 28% of them were ethnic Malays, 50% Chinese, and 22% Indians. There was a right-sided preponderance of microtia involvement, with the total number of right-sided microtia patients approximately being twice as many as patients with left sided involvement.
Two thirds of the microtia patients in the present study were between 10 to 29 years of age. The ideal timing for external ear reconstruction for microtia should be after the age of 6 years when the ear is around 85%–90% of adult size and the rib cartilage to be harvested for reconstruction still retains the growth potential of its source [12]. Many of the congenital microtia patients in this study presented quite late to our Plastic and Reconstructive Surgery Unit.
Meanwhile, the male to female ratio of microtia patients in the present study was found to be 1.6:1, which was less compared to that found in general microtia population with ratio 2.5:1. This is likely due to the small sample size involved in this study. The ethnic distribution in the present study was unlikely to represent the general Malaysian microtia population. The right-sided preponderance of microtia involvement was consistent with previous studies [78]. Three out of the seventeen true microtia patients in this study (i.e., approximately 17%) had bilateral ear involvement.
The results of the present study showed that there was a significant difference in the number of main branches of STA between the sides of the non-microtic ears and those of the microtic ears (P=0.006). The proportion of ears with 2 main branches, namely the frontal and parietal branches, was lower in the microtia group (45.0%) compared to the non-microtia group (89.5%). This could mean that the lateral and posterior scalp were perfused by a more extensive network of anastomotic vessels supplied by the other arteries to the scalp, namely the posterior auricular, occipital, zygomaticotemporal, supraorbital and supratrochlear arteries.
In terms of the level of branching, we found that the results for both the sides of the microtic and non-microtic ears in our study were in agreement with previous studies (Table 8) which all showed a preponderance of branching level above the zygomatic arch—in our case, 94.1% above and 5.9% on the arch for the non-microtic side, and 100% above the arch for the microtic side. Furthermore, there was no significant difference in the distance of branching point in relation to the upper border of the ipsilateral zygomatic arch between these two groups, where the mean distance for the non-microtia group was 2.39 cm and that of the microtia group was 2.42 cm (P=0.942).
The results of the present study showed that the STA on the side of the microtic ear formed a more acute angle with P1, which was a plane drawn parallel to the ipsilateral zygomatic arch compared to that on the side of the non-microtic ear. The median angle of the STA in relation to P1 was significantly different (P=0.011) between these two groups, with the said angle being lesser by 24.5° on the side of the microtic ear compared to that on the non-microtic side.
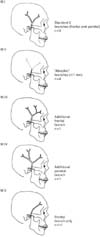
We observed more “single branch” STAs and more antero-superiorly directed STAs on the side of the microtic ears than those on the side of the non-microtic ears. Compared to the classification of the anatomical variations of STA previously put forward by Marano et al. [4] following their analysis of fifty human autopsy specimens, we could see that the type “E” variant with only the frontal branch was the most common (Fig. 3A, B). This information might be particularly useful for any Surgeon who is involved in the use of STA or its branches in vascular anastomosis or as supplying vessels for rotational pedicled fascial flaps.
The results of the present study indicated that the diameters of the STA and its main branches were smaller in both the non-microtia and microtia groups compared to those previously described in the literature for the non-microtic population (Table 9). There are yet to be unequivocal evidence showing ethnic differences in the arterial diameters of STA and any such postulation has to incorporate a uniform technique in measuring the STA diameters of different ethnic groups. Majority of the previous studies involved measurement of the outer diameter of cadaveric specimens of STA pre-infused with liquid medium. Findings in those studies also corresponded with a series of bigger STA diameters compared to those involving measurement of radiographic images of STA in live human subjects (Table 9), which captured the inner diameter of the artery. In this study, measurement of the vessel diameter was taken using digital calipers across the opposite walls of the vessels on 3D reconstructed CTA images. Similarly, for the radiographic part of his study, Stock et al. [3] looked at the selective external carotid angiograms of his study subjects, and measured the diameters of the STA and its two terminal branches. In a Korean study with a sample size of 35 patients, Kim et al. [16] used a digital program (the Aquarius iNtuition, by TeraRecon Inc.) that claimed to produce more accurate and standardized measurements, by negating the alteration in vessel dimensions effected by histographic adjustment in 3D reconstructed CTA images.
In spite of that, comparison between the non-microtia and microtia group within this study itself showed that there was significant difference in their STA diameters (P=0.012). The mean diameter of STA in the non-microtia group was larger than that of the microtia group by 0.4 mm (Table 5). However, the diameters of the frontal and parietal branches of STA showed no significant differences between them for both the non-microtia and the microtia group.
Admittedly, our pilot study was limited to 39 ears. The subjects were selected from the pool of patients managed under the UKMMC Plastic and Reconstructive Surgery Unit, which caters to the central region of peninsular Malaysia, discounting those seen by the private medical facilities. They are likely to be unable to sufficiently represent the general microtia population in this country, with UKMMC being one of a few tertiary medical centres in an urban setting with a plastic and reconstructive surgery service in the country. Besides, in a multiracial county such as Malaysia, we have to take into consideration the possibility that certain ethnic groups might not be as forthcoming as the other in seeking treatment for their medical condition. Nevertheless, this pilot study managed to look into the STA's anatomical variation in part of the microtia population and elicited the differences in the anatomy and course of the STA in this selected group. Recommendations for future studies include having one that is able to incorporate a bigger and more representative size of microtia population, e.g., having a multicenter study across the country. Also, it is crucial that any such study should be able to utilize a standardized and highly accurate mode of measuring the anatomical parameters derived from a 3D reconstructed CTA imaging.
The well-known variation in the anatomy and course of the STA makes it paramount to ascertain these variations in the microtia patients prior to surgical planning in order to minimize any post-operative complications. The scope of benefits of ascertaining the anatomical variation of STA extends well beyond the safer auricular reconstruction for microtia patients alone, as the STA is also widely used as the arterial supply for pedicled fascia flaps in scalp and facial reconstruction. It is also increasingly utilized during bypass surgery in the treatment of ischaemic cerebrovascular diseases. The present pilot descriptive study also demonstrated the anatomical variations that exist in the STA caliber and distributions amongst the microtia population. The results of the present study may provide better understanding of the STA anatomy and its variants in this population which may be beneficial in the surgical planning involving the use of a pedicled temporoparietal fascial flap.
Figures and Tables
Fig. 1
(A) 3D reconstruction of external carotid computed tomographic angiography. (B) Measurement of the diameter of superficial temporal artery (STA) and its main branches. (C) Measurement of the angle of STA and its main branches in relation to a plane drawn parallel to the upper border of the ipsilateral zygomatic arch. (D) Measurement of the distance and level of STA branching point in relation to the upper border of the ipsilateral zygomatic arch.
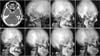
Fig. 3
(A) Classification of superficial temporal artery (STA) by anatomic variations in normal population, according to Marano et al. [4]. Adapted from Marano et al. Neurosurgery 1985;16:786-90 [4], with permission from Wolters Kluwer. (B) Preponderance of different anatomical variants of STA in the microtia population compared to that described by Marano et al. [4] in the normal population. Note that the main variants found in this study is the type “E” variants, i.e., STA with frontal branch only. Also, it was noted that the mean diameters of the STA and its branches were smaller in the microtia population compared to the normal population described by Marano et al. [4].

Table 1
Comparison of presence or absence of STA between the side of the non-microtic and microtic ears

| Non-microtia | Microtia | P-value | |
|---|---|---|---|
| STA | 19/19 | 20/20 | 1.000a) |
| Absent | 0/19 (0.0) | 1/20 (4.8) | |
| Present | 19/19 (100.0) | 20/20 (95.2) |
Table 2
Comparison of the level of branching of STA between the side of the non-microtic and microtic ears

| Non-microtia | Microtia | P-value | |
|---|---|---|---|
| Level of branching in relation to zygomatic arch | 17/19 | 9/20 | 1.000a) |
| Above | 16/17 (94.1) | 9/9 (100.0) | |
| On | 1/17 (5.9) | 0/10 (0.0) |
Table 3
Comparison of number of STA branch(es) between the side of the non-microtic and microtic ears

| Non-microtia | Microtia | P-value | |
|---|---|---|---|
| No. of STA main branches | 19/19 | 20/20 | 0.006a) |
| 1 | 2/19 (10.5) | 11/20 (55.0) | |
| 2 | 17/19 (89.5) | 9/20 (45.0) |
Table 4
Comparison of the distance of STA branching point from the upper border of the ipsilateral zygomatic arch, between the side of the non-microtic and microtic ears

| Non-microtia | Microtia | P-value | |||
|---|---|---|---|---|---|
| No. | Mean±SD | No. | Mean±SD | ||
| Distance of branching point in relation to upper border of the ipsilateral zygomatic arch (cm) | 17/19 | 2.39±1.20 | 9/20 | 2.42±0.96 | 0.942a) |
Table 5
Comparison of diameters of STA and those of its main branches between the side of the non-microtic and microtic ears

Table 6
Comparison of the angles of STA and those of its main branches between the side of the non-microtic and microtic ears

Table 7
Comparison of the number of terminating root of STA/number of terminating branches of its main branch, between the side of the non-microtic and microtic ears

Table 8
Level of STA bifurcation in relation to zygomatic arch

| Author | Above the ZA (%) | On the ZA (%) | Below the ZA (%) |
|---|---|---|---|
| Marano et al. [4] (cadaveric) | 95.7 | 4.3 | N/D |
| Pinar and Govsa [14] (cadaveric) | 74.1 | 22.2 | N/D |
| Chen et al. [5] (cadaveric) | 86.5 | 3.8 | 9.6 |
| Tayfur et al. [15] (cadaveric) | 62 | N/D | 38 |
| Stock et al. [3] (radiological) | 60.0 | 32.0 | 8.0 |
| Kim et al. [16] (radiological) | 84.1 | 8.7 | 7.2 |
| Current study (radiological) | 94.1 (non-microtia) | 5.9 (non-microtia) | 0.0 (non-microtia) |
| 100.0 (microtia) | 0.0 (microtia) | 0.0 (microtia) |
Table 9
Diameters of STA and its main branches (mm)

| Author | STA diameter | Frontal branch diameter | Parietal branch diameter | |||
|---|---|---|---|---|---|---|
| Non-microtia | Microtia | Non-microtia | Microtia | Non-microtia | Microtia | |
| Marano et al. [4] (cadaveric) | 2.2 | - | N/D | - | N/D | - |
| Pinar and Govsa [14] (cadaveric) | 2.7±0.5 | - | 2.1±0.5 | - | 1.8±0.5 | - |
| Chen et al. [5] (cadaveric) | 2.1±0.5 | - | 1.6±0.2 | - | 1.7±0.2 | - |
| Tayfur et al. [15] (cadaveric) | 2.5 | - | 1.8 | - | 1.8 | - |
| Stock et al. [3] (cadaveric) | 2.0±0.3 | - | 1.7±0.5 | - | 1.8±0.3 | - |
| Stock et al. [3] (radiological) | 1.9±0.7 | - | 1.4±0.4 | - | 1.3±0.5 | - |
| Kim et al. [16] (radiological) | 1.8±0.5 | - | 1.4±0.4 | - | 1.4±0.5 | - |
| Current study (radiological) | 1.6±0.4 | 1.2±0.4 | 1.1±0.3 | 1.0±0.2 | 1.1±0.2 | 1.0±0.2 |
References
1. Gardner ED, Gray DJ, O'Rahilly R. Anatomy: a regional study of human structure. 4th ed. Philadelphia, PA: W.B. Saunders;1975.
2. Abul-Hassan HS, von Drasek Ascher G, Acland RD. Surgical anatomy and blood supply of the fascial layers of the temporal region. Plast Reconstr Surg. 1986; 77:17–28.
3. Stock AL, Collins HP, Davidson TM. Anatomy of the superficial temporal artery. Head Neck Surg. 1980; 2:466–469.
4. Marano SR, Fischer DW, Gaines C, Sonntag VK. Anatomical study of the superficial temporal artery. Neurosurgery. 1985; 16:786–790.
5. Chen TH, Chen CH, Shyu JF, Wu CW, Lui WY, Liu JC. Distribution of the superficial temporal artery in the Chinese adult. Plast Reconstr Surg. 1999; 104:1276–1279.
6. Yamada A, Ueda K, Yorozuya-Shibazaki R. External ear reconstruction in hemifacial microsomia. J Craniofac Surg. 2009; 20:Suppl 2. 1787–1793.
7. Melnick M, Myrianthopoulos NC, Paul NW. External ear malformations: epidemiology, genetics, and natural history. Birth Defects Orig Artic Ser. 1979; 15:i–ix. 1–140.
8. Kelley PE, Scholes MA. Microtia and congenital aural atresia. Otolaryngol Clin North Am. 2007; 40:61–80.
9. Brent B. The correction of mi-rotia with autogenous cartilage grafts: I. The classic deformity? Plast Reconstr Surg. 1980; 66:1–12.
10. Nagata S. A new method of total reconstruction of the auricle for microtia. Plast Reconstr Surg. 1993; 92:187–201.
11. Tanzer RC. Total reconstruction of the auricle. The evolution of a plan of treatment. Plast Reconstr Surg. 1971; 47:523–533.
12. Brent B. The correction of microtia with autogenous cartilage grafts: II. Atypical and complex deformities. Plast Reconstr Surg. 1980; 66:13–21.
13. Park C, Lew DH, Yoo WM. An analysis of 123 temporoparietal fascial flaps: anatomic and clinical considerations in total auricular reconstruction. Plast Reconstr Surg. 1999; 104:1295–1306.
14. Pinar YA, Govsa F. Anatomy of the superficial temporal artery and its branches: its importance for surgery. Surg Radiol Anat. 2006; 28:248–253.
15. Tayfur V, Edizer M, Magden O. Anatomic bases of superficial temporal artery and temporal branch of facial nerve. J Craniofac Surg. 2010; 21:1945–1947.
16. Kim BS, Jung YJ, Chang CH, Choi BY. The anatomy of the superficial temporal artery in adult koreans using 3-dimensional computed tomographic angiogram: clinical research. J Cerebrovasc Endovasc Neurosurg. 2013; 15:145–151.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download