1. Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen HU, Kendler KS. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994; 51:8–19.
2. Ansorge MS, Hen R, Gingrich JA. Neurodevelopmental origins of depressive disorders. Curr Opin Pharmacol. 2007; 7:8–17.
3. Gross C, Hen R. The developmental origins of anxiety. Nat Rev Neurosci. 2004; 5:545–552.
4. Leonardo ED, Hen R. Anxiety as a developmental disorder. Neuropsychopharmacology. 2008; 33:134–140.
5. Argyropoulos SV, Sandford JJ, Nutt DJ. The psychobiology of anxiolytic drug. Part 2: Pharmacological treatments of anxiety. Pharmacol Ther. 2000; 88:213–227.
6. Zohar J, Westenberg HG. Anxiety disorders: a review of tricyclic antidepressants and selective serotonin reuptake inhibitors. Acta Psychiatr Scand Suppl. 2000; 403:39–49.
7. Millan MJ. The neurobiology and control of anxious states. Prog Neurobiol. 2003; 70:83–244.
8. Cohen H. Anxiolytic effect and memory improvement in rats by antisense oligodeoxynucleotide to 5-hydroxytryptamine-2A precursor protein. Depress Anxiety. 2005; 22:84–93.
9. Weisstaub NV, Zhou M, Lira A, Lambe E, González-Maeso J, Hornung JP, Sibille E, Underwood M, Itohara S, Dauer WT, Ansorge MS, Morelli E, Mann JJ, Toth M, Aghajanian G, Sealfon SC, Hen R, Gingrich JA. Cortical 5-HT2A receptor signaling modulates anxiety-like behaviors in mice. Science. 2006; 313:536–540.
10. Hurlemann R, Schlaepfer TE, Matusch A, Reich H, Shah NJ, Zilles K, Maier W, Bauer A. Reduced 5-HT(2A) receptor signaling following selective bilateral amygdala damage. Soc Cogn Affect Neurosci. 2009; 4:79–84.
11. Vermeire ST, Audenaert KR, Dobbeleir AA, De Meester RH, De Vos FJ, Peremans KY. Evaluation of the brain 5-HT2A receptor binding index in dogs with anxiety disorders, measured with 123I-5I-R91150 and SPECT. J Nucl Med. 2009; 50:284–289.
12. Benyamina A, Naassila M, Bourin M. Potential role of cortical 5-HT(2A) receptors in the anxiolytic action of cyamemazine in benzodiazepine withdrawal. Psychiatry Res. 2012; 198:307–312.
13. Heisler LK, Zhou L, Bajwa P, Hsu J, Tecott LH. Serotonin 5-HT(2C) receptors regulate anxiety-like behavior. Genes Brain Behav. 2007; 6:491–496.
14. Stein DJ, Ipser JC, Balkom AJ. Pharmacotherapy for social phobia. Cochrane Database Syst Rev. 2004; (4):CD001206.
15. Fernandez M, Pissiota A, Frans O, von Knorring L, Fischer H, Fredrikson M. Brain function in a patient with torture related post-traumatic stress disorder before and after fluoxetine treatment: a positron emission tomography provocation study. Neurosci Lett. 2001; 297:101–104.
16. Nic Dhonnchadha BA, Hascoët M, Jolliet P, Bourin M. Evidence for a 5-HT2A receptor mode of action in the anxiolytic-like properties of DOI in mice. Behav Brain Res. 2003; 147:175–184.
17. Adamec R, Creamer K, Bartoszyk GD, Burton P. Prophylactic and therapeutic effects of acute systemic injections of EMD 281014, a selective serotonin 2A receptor antagonist on anxiety induced by predator stress in rats. Eur J Pharmacol. 2004; 504:79–96.
18. Westenberg HG, Liebowitz MR. Overview of panic and social anxiety disorders. J Clin Psychiatry. 2004; 65:Suppl 14. 22–26.
19. Holmes A. Genetic variation in cortico-amygdala serotonin function and risk for stress-related disease. Neurosci Biobehav Rev. 2008; 32:1293–1314.
20. Connell J, Graeff F, Guimaraes F, Hellewell JS, Hetem LA, Deakin JF. 5-HT2 receptors and anxiety. Behav Pharmacol. 1995; 6:35.
21. Griebel G. 5-Hydroxytryptamine-interacting drugs in animal models of anxiety disorders: more than 30 years of research. Pharmacol Ther. 1995; 65:319–395.
22. Nic Dhonnchadha BA, Bourin M, Hascoët M. Anxiolytic-like effects of 5-HT2 ligands on three mouse models of anxiety. Behav Brain Res. 2003; 140:203–214.
23. Millan MJ, Brocco M, Gobert A, Dekeyne A. Anxiolytic properties of agomelatine, an antidepressant with melatoninergic and serotonergic properties: role of 5-HT2C receptor blockade. Psychopharmacology (Berl). 2005; 177:448–458.
24. Masse F, Petit-Demouliere B, Dubois I, Hascoët M, Bourin M. Anxiolytic-like effects of DOI microinjections into the hippocampus (but not the amygdala nor the PAG) in the mice four plates test. Behav Brain Res. 2008; 188:291–297.
25. de Paula Soares V, Zangrossi H Jr. Stimulation of 5-HT1A or 5-HT2A receptors in the ventrolateral periaqueductal gray causes anxiolytic-, but not panicolytic-like effect in rats. Behav Brain Res. 2009; 197:178–185.
26. Bombardi C. Neuronal localization of 5-HT2A receptor immunoreactivity in the rat hippocampal region. Brain Res Bull. 2012; 87:259–273.
27. Benekareddy M, Vadodaria KC, Nair AR, Vaidya VA. Postnatal serotonin type 2 receptor blockade prevents the emergence of anxiety behavior, dysregulated stress-induced immediate early gene responses, and specific transcriptional changes that arise following early life stress. Biol Psychiatry. 2011; 70:1024–1032.
28. Schmidt PJ, Rubinow DR. Sex hormones and mood in the perimenopause. Ann N Y Acad Sci. 2009; 1179:70–85.
29. Inagaki T, Gautreaux C, Luine V. Acute estrogen treatment facilitates recognition memory consolidation and alters monoamine levels in memory-related brain areas. Horm Behav. 2010; 58:415–426.
30. Biegon A, Bercovitz H, Samuel D. Serotonin receptor concentration during the estrous cycle of the rat. Brain Res. 1980; 187:221–225.
31. Biegon A, McEwen BS. Modulation by estradiol of serotonin receptors in brain. J Neurosci. 1982; 2:199–205.
32. Zheng P. Neuroactive steroid regulation of neurotransmitter release in the CNS: action, mechanism and possible significance. Prog Neurobiol. 2009; 89:134–152.
33. Berumen LC, Sánchez-Ramos MA, García-Servín M, Martínez-Torres A, Rodríguez A, García-Alcocer G. Effects of estradiol on 5-HT5A and 5-HT2C receptor immunolabeling in rat hippocampus. J Life Sci. 2011; 5:884–889.
34. Bethea CL, Coleman K, Phu K, Reddy AP, Phu A. Relationships between androgens, serotonin gene expression and innervation in male macaques. Neuroscience. 2014; 274:341–356.
35. McEwen BS, Magarinos AM, Reagan LP. Studies of hormone action in the hippocampal formation: possible relevance to depression and diabetes. J Psychosom Res. 2002; 53:883–890.
36. ter Horst GJ. Estrogen in the limbic system. Vitam Horm. 2010; 82:319–338.
37. Carrier N, Kabbaj M. Extracellular signal-regulated kinase 2 signaling in the hippocampal dentate gyrus mediates the antidepressant effects of testosterone. Biol Psychiatry. 2012; 71:642–651.
38. Xu X, Dong F, Yang Y, Wang Y, Wang R, Shen X. Sex-specific effects of long-term exposure to bisphenol-A on anxiety- and depression-like behaviors in adult mice. Chemosphere. 2015; 120:258–266.
39. Filová B, Domonkos E, Borbélyová V, Bábíčková J, Tóthová Ľ, Ostatníková D, Celec P, Hodosy J. Does the non-genomic effect of testosterone on social anxiety require the presence of a classical steroid receptor? Acta Neurobiol Exp (Wars). 2015; 75:457–461.
40. Edinger KL, Frye CA. Testosterone's analgesic, anxiolytic, and cognitive-enhancing effects may be due in part to actions of its 5alpha-reduced metabolites in the hippocampus. Behav Neurosci. 2004; 118:1352–1364.
41. Fernández-Guasti A, Martínez-Mota L. Anxiolytic-like actions of testosterone in the burying behavior test: role of androgen and GABA-benzodiazepine receptors. Psychoneuroendocrinology. 2005; 30:762–770.
42. Carrier N, Saland SK, Duclot F, He H, Mercer R, Kabbaj M. The anxiolytic and antidepressant-like effects of testosterone and estrogen in gonadectomized male rats. Biol Psychiatry. 2015; 78:259–269.
43. Lagunas N, Calmarza-Font I, Grassi D, Garcia-Segura LM. Estrogen receptor ligands counteract cognitive deficits caused by androgen deprivation in male rats. Horm Behav. 2011; 59:581–584.
44. Leranth C, Hajszan T, MacLusky NJ. Androgens increase spine synapse density in the CA1 hippocampal subfield of ovariectomized female rats. J Neurosci. 2004; 24:495–499.
45. Ooishi Y, Kawato S, Hojo Y, Hatanaka Y, Higo S, Murakami G, Komatsuzaki Y, Ogiue-Ikeda M, Kimoto T, Mukai H. Modulation of synaptic plasticity in the hippocampus by hippocampus-derived estrogen and androgen. J Steroid Biochem Mol Biol. 2012; 131:37–51.
46. Bancroft JD, Gamble M. Theory and practice of histological techniques. 5th ed. London: Churchill Livingstone;2002.
47. Bialek M, Zaremba P, Borowicz KK, Czuczwar SJ. Neuroprotective role of testosterone in the nervous system. Pol J Pharmacol. 2004; 56:509–518.
48. Gold SM, Voskuhl RR. Testosterone replacement therapy for the treatment of neurological and neuropsychiatric disorders. Curr Opin Investig Drugs. 2006; 7:625–630.
49. Pike CJ, Rosario ER, Nguyen TV. Androgens, aging, and Alzheimer's disease. Endocrine. 2006; 29:233–241.
50. Bombardi C, Di Giovanni G. Functional anatomy of 5-HT2A receptors in the amygdala and hippocampal complex: relevance to memory functions. Exp Brain Res. 2013; 230:427–439.
51. Karimi S, Jahanshahi M, Golalipour MJ. The effect of MDMA-induced anxiety on neuronal apoptosis in adult male rats' hippocampus. Folia Biol (Praha). 2014; 60:187–191.
52. Carrier N, Kabbaj M. Testosterone and imipramine have antidepressant effects in socially isolated male but not female rats. Horm Behav. 2012; 61:678–685.
53. Roohbakhsh A, Moghaddam AH, Delfan KM. Anxiolytic-like effect of testosterone in male rats: GABAC receptors are not involved. Iran J Basic Med Sci. 2011; 14:376–382.
54. Jahanshahi M, Nickmahzar EG, Seif-hoseini S, Babakordi F, Moharreri A. Scopolamine reduces the density of M1 muscarinic neurons in rats' hippocampus. Int J Morphol. 2013; 31:1227–1232.
55. Bancroft JD, Gamble M. Theory and practice of histological techniques. 6th ed. London: Churchill Livingstone;2008.
56. Jahanshahi M, Sadeghi Y, Hosseini A. Estimation of astrocyte number in different subfield of rat hippocampus. Pak J Biol Sci. 2006; 9:1595–1597.
57. Emamian S, Naghdi N, Sepehri H, Jahanshahi M, Sadeghi Y, Choopani S. Learning impairment caused by intra-CA1 microinjection of testosterone increases the number of astrocytes. Behav Brain Res. 2010; 208:30–37.
58. Khodaie B, Lotfinia AA, Ahmadi M, Lotfinia M, Jafarian M, Karimzadeh F, Coulon P, Gorji A. Structural and functional effects of social isolation on the hippocampus of rats with traumatic brain injury. Behav Brain Res. 2015; 278:55–65.
59. Jahanshahi M, Nickmahzar EG, Babakordi F. Effect of Gingko biloba extract on scopolamine-induced apoptosis in the hippocampus of rats. Anat Sci Int. 2013; 88:217–222.
60. McDermott CM, Liu D, Schrader LA. Role of gonadal hormones in anxiety and fear memory formation and inhibition in male mice. Physiol Behav. 2012; 105:1168–1174.
61. Frye CA, Seliga AM. Testosterone increases analgesia, anxiolysis, and cognitive performance of male rats. Cogn Affect Behav Neurosci. 2001; 1:371–381.
62. Khakpai F. The effect of opiodergic system and testosterone on anxiety behavior in gonadectomized rats. Behav Brain Res. 2014; 263:9–15.
63. Cooper MA, Ritchie EC. Testosterone replacement therapy for anxiety. Am J Psychiatry. 2000; 157:1884.
64. Hodosy J, Zelmanová D, Majzúnová M, Filová B, Malinová M, Ostatníková D, Celec P. The anxiolytic effect of testosterone in the rat is mediated via the androgen receptor. Pharmacol Biochem Behav. 2012; 102:191–195.
65. Frye CA, Edinger K, Sumida K. Androgen administration to aged male mice increases anti-anxiety behavior and enhances cognitive performance. Neuropsychopharmacology. 2008; 33:1049–1061.
66. Bitran D, Kellogg CK, Hilvers RJ. Treatment with an anabolic-androgenic steroid affects anxiety-related behavior and alters the sensitivity of cortical GABA A receptors in the rat. Horm Behav. 1993; 27:568–583.
67. Edinger KL, Frye CA. Testosterone's anti-anxiety and analgesic effects may be due in part to actions of its 5alpha-reduced metabolites in the hippocampus. Psychoneuroendocrinology. 2005; 30:418–430.
68. Del Cerro S, Garcia-Estrada J, Garcia-Segura LM. Neuroactive steroids regulate astroglia morphology in hippocampal cultures from adult rats. Glia. 1995; 14:65–71.
69. Day JR, Laping NJ, McNeill TH, Schreiber SS, Pasinetti G, Finch CE. Castration enhances expression of glial fibrillary acidic protein and sulfated glycoprotein-2 in the intact and lesion-altered hippocampus of the adult male rat. Mol Endocrinol. 1990; 4:1995–2002.
70. Garcia-Segura LM, Chowen JA, Dueñas M, Parducz A, Naftolin F. Gonadal steroids and astroglial plasticity. Cell Mol Neurobiol. 1996; 16:225–237.
71. Barreto-Estrada JL, Barreto J, Fortis-Santiago Y, Rivera-Ramos I, Fortis-Santiago A, Jorge JC. Modulation of affect after chronic exposure to the anabolic steroid 17alpha-methyltestosterone in adult mice. Behav Neurosci. 2004; 118:1071–1079.
72. Ageta H, Murayama A, Migishima R, Kida S, Tsuchida K, Yokoyama M, Inokuchi K. Activin in the brain modulates anxiety-related behavior and adult neurogenesis. PLoS One. 2008; 3:e1869.
73. Bergami M, Rimondini R, Santi S, Blum R, Götz M, Canossa M. Deletion of TrkB in adult progenitors alters newborn neuron integration into hippocampal circuits and increases anxiety-like behavior. Proc Natl Acad Sci U S A. 2008; 105:15570–15575.
74. Revest JM, Dupret D, Koehl M, Funk-Reiter C, Grosjean N, Piazza PV, Abrous DN. Adult hippocampal neurogenesis is involved in anxiety-related behaviors. Mol Psychiatry. 2009; 14:959–967.
75. Sah A, Schmuckermair C, Sartori SB, Gaburro S, Kandasamy M, Irschick R, Klimaschewski L, Landgraf R, Aigner L, Singewald N. Anxiety- rather than depression-like behavior is associated with adult neurogenesis in a female mouse model of higher trait anxiety- and comorbid depression-like behavior. Transl Psychiatry. 2012; 2:e171.
76. Benice TS, Raber J. Castration and training in a spatial task alter the number of immature neurons in the hippocampus of male mice. Brain Res. 2010; 1329:21–29.
77. Spritzer MD, Galea LA. Testosterone and dihydrotestosterone, but not estradiol, enhance survival of new hippocampal neurons in adult male rats. Dev Neurobiol. 2007; 67:1321–1333.
78. Wainwright SR, Lieblich SE, Galea LA. Hypogonadism predisposes males to the development of behavioural and neuroplastic depressive phenotypes. Psychoneuroendocrinology. 2011; 36:1327–1341.
79. Spritzer MD, Ibler E, Inglis W, Curtis MG. Testosterone and social isolation influence adult neurogenesis in the dentate gyrus of male rats. Neuroscience. 2011; 195:180–190.
80. Allen KM, Fung SJ, Rothmond DA, Noble PL, Weickert CS. Gonadectomy increases neurogenesis in the male adolescent rhesus macaque hippocampus. Hippocampus. 2014; 24:225–238.
81. Galea LA. Gonadal hormone modulation of neurogenesis in the dentate gyrus of adult male and female rodents. Brain Res Rev. 2008; 57:332–341.
82. Buwalda B, van der Borght K, Koolhaas JM, McEwen BS. Testosterone decrease does not play a major role in the suppression of hippocampal cell proliferation following social defeat stress in rats. Physiol Behav. 2010; 101:719–725.
83. Allen KM, Purves-Tyson TD, Fung SJ, Shannon Weickert C. The effect of adolescent testosterone on hippocampal BDNF and TrkB mRNA expression: relationship with cell proliferation. BMC Neurosci. 2015; 16:4.
84. Farinetti A, Tomasi S, Foglio B, Ferraris A, Ponti G, Gotti S, Peretto P, Panzica GC. Testosterone and estradiol differentially affect cell proliferation in the subventricular zone of young adult gonadectomized male and female rats. Neuroscience. 2015; 286:162–170.
85. Cyr M, Bossé R, Di Paolo T. Gonadal hormones modulate 5-hydroxytryptamine2A receptors: emphasis on the rat frontal cortex. Neuroscience. 1998; 83:829–836.
86. Sumner BE, Fink G. Testosterone as well as estrogen increases serotonin2A receptor mRNA and binding site densities in the male rat brain. Brain Res Mol Brain Res. 1998; 59:205–214.
87. Fink G, Sumner B, Rosie R, Wilson H, McQueen J. Androgen actions on central serotonin neurotransmission: relevance for mood, mental state and memory. Behav Brain Res. 1999; 105:53–68.
88. Summer BE, Fink G. Estrogen increases the density of 5-hydroxytryptamine2A receptors in cerebral cortex and nucleus accumbens in the female rat. J Steroid Biochem Mol Biol. 1995; 54:15–20.
89. Kugaya A, Epperson CN, Zoghbi S, van Dyck CH, Hou Y, Fujita M, Staley JK, Garg PK, Seibyl JP, Innis RB. Increase in prefrontal cortex serotonin 2A receptors following estrogen treatment in postmenopausal women. Am J Psychiatry. 2003; 160:1522–1524.
90. Moses EL, Drevets WC, Smith G, Mathis CA, Kalro BN, Butters MA, Leondires MP, Greer PJ, Lopresti B, Loucks TL, Berga SL. Effects of estradiol and progesterone administration on human serotonin 2A receptor binding: a PET study. Biol Psychiatry. 2000; 48:854–860.
91. Moses-Kolko EL, Berga SL, Greer PJ, Smith G, Cidis Meltzer C, Drevets WC. Widespread increases of cortical serotonin type 2A receptor availability after hormone therapy in euthymic postmenopausal women. Fertil Steril. 2003; 80:554–559.
92. Birzniece V, Johansson IM, Wang MD, Bäckström T, Olsson T. Ovarian hormone effects on 5-hydroxytryptamine(2A) and 5-hydroxytryptamine(2C) receptor mRNA expression in the ventral hippocampus and frontal cortex of female rats. Neurosci Lett. 2002; 319:157–161.
93. Bethea CL, Lu NZ, Gundlah C, Streicher JM. Diverse actions of ovarian steroids in the serotonin neural system. Front Neuroendocrinol. 2002; 23:41–100.
94. Höfer P, Lanzenberger R, Kasper S. Testosterone in the brain: neuroimaging findings and the potential role for neuropsychopharmacology. Eur Neuropsychopharmacol. 2013; 23:79–88.
95. Bruning CA, Prigol M, Roehrs JA, Nogueira CW, Zeni G. Involvement of the serotonergic system in the anxiolytic-like effect caused by m-trifluoromethyl-diphenyl diselenide in mice. Behav Brain Res. 2009; 205:511–517.
96. Zhang L, Ma W, Barker JL, Rubinow DR. Sex differences in expression of serotonin receptors (subtypes 1A and 2A) in rat brain: a possible role of testosterone. Neuroscience. 1999; 94:251–259.
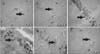
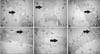
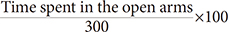 ×100
×100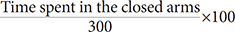 ×100
×100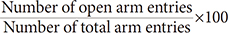 ×100
×100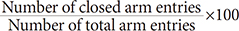 ×100
×100



 PDF
PDF ePub
ePub Citation
Citation Print
Print









 XML Download
XML Download