Abstract
The superior laryngeal artery is the principal artery supplying the laryngeal mucosa, musculature, and glands. Knowledge of variations in the origin of superior laryngeal artery could prove to be very useful during reconstructive surgeries of the larynx, partial laryngectomy, laryngeal transplantation, and also during procedures like super-selective intra-arterial chemotherapy for laryngeal and hypolaryngeal cancers. However, relatively few studies have been done on the superior laryngeal artery in comparison to its clinical importance. The present study was aimed at documenting the prevalence of variable origin of the superior laryngeal artery within the carotid triangle. Sixty hemi-necks obtained from 30 South Indian cadavers were dissected and studied for variations in the origin of superior laryngeal artery. It was observed that the superior laryngeal artery took origin from superior thyroid in 91.7% cases. Variable origin from the external carotid artery was noted in 5% cases. The superior laryngeal artery was found to arise from the lingual artery in one case alone (1.7%). In addition to the above findings, a very rare variation of superior laryngeal artery arising from the ascending pharyngeal (1.7%) was also observed in the hemi-neck of one cadaver. All the variations that were observed were unilateral and on the left side. These findings may help provide further insight to the anatomists, radiologists and surgeons and can help improve performances during surgical manipulations of the larynx.
The superior laryngeal artery is a major source of blood supply to the larynx. As per conventional textbooks of anatomy, the superior laryngeal artery (SLA) is derived from the superior thyroid artery (STA). Though variations in the origin of SLA are not very common, the variations when present can acquire great importance in surgical procedures of the larynx like partial laryngectomy, reconstruction surgery and laryngeal transplantation [12]. Knowledge of SLA variations can also be helpful to clinicians for super-selective intra-arterial infusion chemotherapy for laryngeal and hypopharyngeal cancers, as well as in successful radical neck dissection for minimizing postoperative complications in a bloodless surgery [34]. In view of its considerable clinical importance, it is desirable to have a thorough understanding of variant origins of SLA. In the current literature, only very few studies are reported and hence the purpose of this research was to document variations in the origin of SLA in the carotid triangle.
The study was carried out on 60 hemi-necks of 30 formalin embalmed cadavers (25 male and 5 female). All the cadavers were aged above 50 years (range being 50–80 years). The carotid triangles were exposed bilaterally as per instructions in the Cunningham's Manual of Anatomy [5] and the origin of the SLA was noted. The arteries were traced up to the thyrohyoid membrane which they pierced along with internal laryngeal nerve. The observations were documented by digital photography. The data were tabulated and the percentage of specimens with variations in the origin of SLA were computed and analyzed.
Out of the 60 hemi-necks studied, the SLA arose from the STA in 55/60 cases (91.7%). In the remaining cases, SLA took origin from the external carotid artery (ECA) in 3/60 cases (5%) (Fig. 1). In all the 3 cases, SLA took origin from ECA above the level of STA and followed a descending course towards the lower part of the thyrohyoid membrane. Variant origin of the SLA from lingual artery (LA) was observed in a single case 1/60 (1.7%) (Fig. 2). A very rare variant origin of the SLA arising from ascending pharyngeal artery (APA) was also noted in one case, 1/60 (1.7%) (Fig. 3). The schematic illustration of our results is depicted in Fig. 4. SLA was not found taking origin from the facial arteries or common trunks between facial, lingual or thyroid arteries. All the variant origins were noted on the left side and in males (Table 1).
The SLA being the major source of blood supply to the human larynx, knowledge of its detailed anatomy and possible variations is needed to prevent vascular damage and bleeding during various surgical procedures. Its importance is highlighted by the fact, that “bilateral perfusion of the entire larynx transplant, including laryngeal and epiglottic mucosa, would occur after revascularization of a single SLA” [1].
The commonest origin of SLA is from STA, the reported range being 68% to 92.7% [36789]. In the present study we observed the origin of SLA from STA in 91.7% cases. In 5% cases, SLA arose from ECA. This is more or less in agreement with the studies by Terayama et al. [3], Lang et al. [8], and Ozgur et al. [10], but differ from that of Rusu et al. [6] who reported a higher incidence of 32%. There are also case reports of SLA taking origin from ECA. Muralimanju et al. [11] reported a case of left SLA taking origin from ECA. The STA in the same case took origin from common carotid artery (CCA) 2 cm before its bifurcation.
Rarely, SLA may arise from APA [31213]. A single such case was noted in this study. Variable origins of SLA from the facial and lingual arteries have also been reported in the literature [12]. Terayama et al. [3] observed SLA arising from LA in one out of 96 carotid angiograms. The present study shows a similar finding in which SLA arose from LA in one case. Origin from facial artery was not observed in our study. There are rare reports of SLA arising from common trunks such as linguofacial trunks [4] and that of a common trunk taking origin from ECA giving rise to SLA and inferior thyroid arteries [14]. Motwani and Jhajhria [15] noticed a rare common trunk arising from the anterior surface of right ECA just above the carotid bifurcation. The common trunk was found dividing into five branches i.e., infrahyoid, superior laryngeal, superior thyroid, cricothyroid, and sternocleidomastoid artery. However, no such common trunk origin of SLA was found in our study.
Vazquez et al. [7] was the first to classify the origin of SLA into 4 types. Type I from STA being commonest (78%), type II from ECA (9%), type III from CCA common carotid artery (5%), type IV from the carotid bifurcation (4%), and absent SLA (4%). We did not encounter any case of origin of SLA from common carotid or carotid bifurcation in the present study. Absence of SLA was not noticed in any of the cases studied.
Nayak et al. [4] classified variations in the origin of SLA into four types: type I, from the STA (79.6%); type II, from the LA (1.3%); type III, directly from the external carotid artery (5.4%); and type IV, from the linguo-facial trunk (1.3%). Applying the classification criteria of Nayak to this study, type I origin was found in 91.7%, type II in 1.7%, and type III in 5% cases, respectively. Type IV origin from the linguofacial trunk was not observed by us. A comparison between the present study and other related studies is depicted in Table 2.
During development, the ECA sprouts from the aortic sac close to the ventral end of the third arch artery and extends cranially during further growth [16]. As the ECA emerges from the base of the third arch, the stem of ventral pharyngeal artery is incorporated within it. The maxillary branch of ventral pharyngeal artery communicates with the common trunk of origin of maxillary and mandibular branches of the stapedial artery and annex their branches [16]. This would result in the final branching pattern of ECA. The failure of proper synchronization of signals involved in annexation and regression will lead to the variations of branching pattern of the ECA [17].
In the present study, superior laryngeal arteries were traced up to the thyrohyoid membrane which they were found piercing along with internal laryngeal nerve. However, there are reports in literature [1819] where superior laryngeal arteries were found passing through ‘foramen thyroideaum’ (FT) usually on the upper part of lamina on the thyroid cartilage behind the oblique line. According to Léon et al. [20] FT is due to the result of failure in the chondrification of mesenchymal elements derived from the 4th and 6th arches, (from which the thyroid cartilage develops) associated with the presence of vessels and/or nerves proceeding from the superior laryngeal pedicle.
Variant origin of STA and SLA from the carotid arterial system significantly increases the possibility of their misidentification during surgery [7] especially considering the fact that variations are not uncommon. Hence, detailed knowledge of the variant anatomy will be helpful in procedures such as partial laryngectomy, reconstructive laryngeal surgeries, as well as laryngeal transplantation [10]. Awareness of variant anatomy can also be of use during radical neck dissection, and minimizing postoperative complications in a bloodless surgery [4]. Moreover SLA being the principal artery of the larynx, is used for administering chemotherapeutic drugs for treatment of laryngeal cancers, as the drugs administered through the feeding artery directly to the tumour site can achieve a greater therapeutic effect [21]. A good and detailed knowledge of SLA will also ensure a correct decision and a safe approach prior to neuro-radiological procedures involving SLA [6].
Figures and Tables
Fig. 1
Superior laryngeal artery arising as a separate branch from external carotid artery. APA, ascending pharyngeal artery; CCA, common carotid artery; DG, digastric; ECA, external carotid artery; FA, facial artery; HN, hypoglossal nerve; ICA, internal carotid artery; IJV, internal jugular vein; LA, lingual artery; OA, occipital artery; SH, stylohyoid; SLA, superior laryngeal artery; STA, superior thyroid artery; TC, thyroid cartilage; TLN, internal laryngeal nerve.
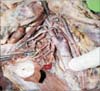
Fig. 2
Superior laryngeal artery arising from lingual artery. CCA, common carotid artery; DG, digastric; ECA, external carotid artery; FA, facial artery; HN, hypoglossal nerve; ICA, internal carotid artery; ILN, internal laryngeal nerve; LA, lingual artery; OA, occipital artery; SH, stylohyoid; SLA, superior laryngeal artery; STA, superior thyroid artery; TC, thyroid cartilage.

Fig. 3
Superior laryngeal artery arising from ascending pharyngeal artery. APA, ascending pharyngeal artery; CCA, common carotid artery; DG, digastric; ECA, external carotid artery; FA, facial artery; HN, hypoglossal nerve; ICA, internal carotid artery; IJV, internal jugular vein; LA, lingual artery; OA, occipital artery; OH-S, omohyoid superior belly; SH, stylohyoid; SLA, superior laryngeal artery; SMG, sub mandibular gland; STA, superior thyroid artery; VN, vagus nerve.
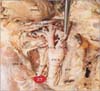
Fig. 4
Schematic representation of variant origins of superior laryngeal artery. (A) From external carotid artery. (B) From lingual artery. (C) From ascending pharyngeal artery.

Table 1
Variant origins of superior laryngeal artery

| No. | Origin of SLA | Side of variant origin | Sex | No. of specimens | Percentage |
|---|---|---|---|---|---|
| 1 | From STA | Left | Male | 55 | 91.7 |
| 2 | From ECA | Left | Male | 3 | 5 |
| 3 | From LA | Left | Male | 1 | 1.7 |
| 4 | From APA | Left | Male | 1 | 1.7 |
Table 2
Comparison between the present study and other reported studies on the variant origin of superior laryngeal artery

| No. | Origin of SLA | Rusu et al. (2007) [6] | Vazquez et al. (2009) [7] | Nayak et al. (2011) [4] | Present study (2016) |
|---|---|---|---|---|---|
| 1 | From the STA | 68 | 78 | 79.6 | 91.7 |
| 2 | From the LA | - | - | 1.3 | 1.7 |
| 3 | Directly from ECA | 32 | 9 | 5.4 | 5 |
| 4 | From the linguo-facial trunk | - | - | 1.3 | - |
| 5 | From the APA | - | - | - | 1.7 |
| 6 | From CCA | - | 5 | - | - |
| 7 | From carotid bifurcation | - | 4 | - | - |
| 8 | Absent | - | 4 | - | - |
References
1. Anthony JP, Argenta P, Trabulsy PP, Lin RY, Mathes SJ. The arterial anatomy of larynx transplantation: microsurgical revascularization of the larynx. Clin Anat. 1996; 9:155–159.
2. Iimura A, Itoh M, Terayama H, Nakamura Y, He G, Kondo Y, Takahashi T, Shimada K. Anatomical study of meandering and functions of human intralaryngeal artery. Okajimas Folia Anat Jpn. 2004; 81:85–92.
3. Terayama N, Sanada J, Matsui O, Kobayashi S, Kawashima H, Yamashiro M, Takanaka T, Kumano T, Yoshizaki T, Furukawa M. Feeding artery of laryngeal and hypopharyngeal cancers: role of the superior thyroid artery in superselective intraarterial chemotherapy. Cardiovasc Intervent Radiol. 2006; 29:536–543.
4. Nayak SR, Krishnamurthy A, Prabhu LV, Potu BK, Bagoji IB, Jiji PJ, Chettiar GK. Variable origin of the superior laryngeal artery and its clinical significance. Al Ameen J Med Sci. 2011; 4:69–74.
5. Romanes GJ. Cunningham's manual of practical anatomy. Vol. 3. Head, neck and brain. 15th ed. New York: Oxford Medical Publications;2005.
6. Rusu MC, Nimigean V, Banu MA, Cergan R, Niculescu V. The morphology and topography of the superior laryngeal artery. Surg Radiol Anat. 2007; 29:653–660.
7. Vázquez T, Cobiella R, Maranillo E, Valderrama FJ, McHanwell S, Parkin I, Sañudo JR. Anatomical variations of the superior thyroid and superior laryngeal arteries. Head Neck. 2009; 31:1078–1085.
8. Lang J, Nachbaur S, Fischer K, Vogel E. The superior laryngeal nerve and the superior laryngeal artery. Acta Anat (Basel). 1987; 130:309–318.
9. Trotoux J, Germain MA, Bruneau X. Vascularization of the larynx. Update of classical anatomic data from an anatomical study of 100 subjects. Ann Otolaryngol Chir Cervicofac. 1986; 103:389–397.
10. Ozgur Z, Govsa F, Celik S, Ozgur T. Clinically relevant variations of the superior thyroid artery: an anatomic guide for surgical neck dissection. Surg Radiol Anat. 2009; 31:151–159.
11. Murlimanju BV, Prabhu LV, Pai MM, Jayaprakash D, Saralaya VV. Variant origins of arteries in the carotid triangle: a case report. Chang Gung Med J. 2012; 35:281–284.
12. Bergman RA, Afifi AK, Miyauchi R. Illustrated encyclopedia of human anatom-ic variation: Opus II: cardiovascular system: alphabetical listing [Internet]. Anatomy Atlas;2016. cited 2016 Nov 30. Available from: http://www.anatomyatlases.org/AnatomicVariants/Cardiovascular/Directory/Alphabetical/S.shtml.
13. Lasjaunias P, Moret J. The ascending pharyngeal artery: normal and pathological radioanatomy. Neuroradiology. 1976; 11:77–82.
14. Billakanti PB, Huban T. Anomalous origin of superior laryngeal artery from external carotid artery: a case report. Int Arch Integr Med. 2015; 2:144–147.
15. Motwani R, Jhajhria SK. Variant branching pattern of superior thyroid artery and its clinical relevance: a case report. J Clin Diagn Res. 2015; 9:AD05–AD06.
16. Standring S, Gray H. Gray's anatomy: the anatomical basis of clinical practice. 40th ed. Edinburgh: Churchill Livingstone/Elsevier;2008.
17. Altmann F. Anomalies of the internal carotid artery and its branches; their embryological and comparative anatomical significance; report of a new case of persistent stapedial artery in man. Laryngoscope. 1947; 57:313–339.
18. Ortug C, Gunduz T, Sam B. The incidence of the foramen thyroideum in Turkish population. Surg Radiol Anat. 2005; 27:491–494.
19. Dehghani-Soltani S, Eftekhar-Vaghefi SH, Babaee A. Case report: an uncom-mon variation of the superior laryngeal artery. Anat Sci. 2016; 13:63–66.
20. León X, Maranillo E, Mirapeix RM, Quer M, Sañudo JR. Foramen thyroideum: a comparative study in embryos, fetuses, and adults. Laryngoscope. 1997; 107:1146–1150.
21. Shimizu T, Sakakura Y, Hattori T, Yamaguchi N, Kubo M, Sakakura K. Superselective intraarterial chemotherapy in combination with irradiation: preliminary report. Am J Otolaryngol. 1990; 11:131–136.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download