Abstract
Dentin is the major part of tooth and formed by odontoblasts. Under the influence of the inner enamel epithelium, odontoblasts differentiate from ectomesenchymal cells of the dental papilla and secrete pre-dentin which then undergo mineralization into dentin. Transforming growth factor-beta (TGF-β)/bone morphogenetic protein (BMP) signaling is essential for dentinogenesis; however, the precise molecular mechanisms remain unclear. To understand the role of TGF-β/BMP signaling in odontoblast differentiation and dentin formation, we generated mice with conditional ablation of Smad4, a key intracellular mediator of TGF-β/BMP signaling, using Osr2 or OC-Cre mice. Here we found the molars of Osr2CreSmad4 mutant mice exhibited impaired odontoblast differentiation, and normal dentin was replaced by ectopic bone-like structure. In Osr2CreSmad4 mutant mice, cell polarity of odontoblast was lost, and the thickness of crown dentin was decreased in later stage compared to wild type. Moreover, the root dentin was also impaired and showed ectopic bone-like structure similar to Osr2CreSmad4 mutant mice. Taken together, our results suggest that Smad4-dependent TGF-β/BMP signaling plays a critical role in odontoblast differentiation and dentin formation during tooth development.
During dentinogenesis, cranial neural crest cell–derived odontoblasts play a role in the secretion of pre-dentin and dentin following terminal differentiation [1]. Dentin is a major component of tooth and is produced by odontoblasts. During the late bell stage of tooth development, odontoblasts differentiate from ectomesenchymal cells of the dental papilla under the influence of the inner dental epithelium [2]. Moreover, odontoblasts then produce extracellular matrix, including collagen type I as well as non-collagenous proteins such as dentin sialophosphoprotein (Dspp), osteocalcin (OC), and dentin matrix protein 1 (Dmp1) which facilitate the mineralization of the dentin matrix [34].
Transforming growth factor-beta (TGF-β) superfamily, insulin-like growth factors, WNTs, fibroblast growth factors, and other kinds of growth factors are involved in differentiation of dental papilla cells into odontoblasts during tooth development [5]. TGF-β is a multifunctional regulator of a variety of cellular functions, including cell proliferation, differentiation, apoptosis and matrix synthesis [6]. TGF-β/bone morphogenetic protein (BMP) signaling has been implicated to have specific roles during tooth development as seen various disturbances with its disruption by tissue-specific targeting of odontoblast and dentin formation. In a previous study, Wnt1-Cre;Tgfbr2 mice have shown delayed odontoblast differentiation and decreased dentin thickness [7]. Moreover, targeted inactivation of Bmp2 or Bmp4 also showed phenotypes of impaired odontoblast maturation and tooth root defects [89]. These data suggested that TGF-β/BMP signaling plays critical role in odontoblast differentiation and dentin formation.
TGF-β superfamily consists of TGF-βs, BMPs, activins, and other related proteins, and TGF-β/BMP signaling transduction pathway maintains cell proliferation, differentiation, apoptosis, migration, and reconstruction of proteins [10]. Smad4, a key mediator of TGF-β/BMP signaling, functions as a multifunctional regulator for cranial neural crest cell migration, proliferation, differentiation, protein reconstruction, and immune response, or other physiological functions [1011] and expresses in oral epithelium and dental mesenchyme during tooth development [12]. To study the functions of Smad4 during full tooth development, tissue-specific gene targeting technology like conditional knockout strategy is essentially required to overcome disadvantage of Smad4-null mice, which is arrested at E7.5–E8.5 with early stage of tooth development [13].
Here, we generated and analyzed the tissue-specific conditional Smad4 disruption mice under the control of Osr2 and OC promoter to investigate the role of TGF-β/BMP signaling in odontoblast differentiation and dentin formation.
All experimental procedures were approved by the animal Welfare Committee of Chonbuk National University. Smad4-floxed allele (Smad4fl/fl), Osr2Ires-Cre, and OC-Cre mice have been previously described [141516]. Tissue specific activities of Osr2Ires-Cre and OC-Cre have been reported in dental mesenchyme and odontoblasts [1516]. Rosa26 (R26R) reporter mice [17] were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). To generate Osr2Ires-Cre:Smad4fl/fl (Osr2CreSmad4) and OC-Cre:Smad4fl/fl (OCCreSmad4) mice, Osr2Ires-Cre:Smad4fl/+ and OC-Cre:Smad4fl/+ (control) mice were crossed with Smad4fl/fl mice, respectively. Genotyping of mice was carried out by allele-specific polymerase chain reaction as previously described using the following oligonucleotide primers: Smad4 floxed (Smad4a, 5'-AAG AGC CAC AGG TCA AGC AG-3'; Smad4b, 5'-GGG CAG CGT AGC ATA TAA GA-3'; Smad4c, 5'-GAC CCA AAC GTC ACC TTC AC-3'), OC-Cre (Cre1, 5'-ATC CGA AAA GAA AAC GTT GA-3'; Cre2, 5'-ATC CAG GTT ACG GAT ATA GT-3'). Osr2Ires-Cre (Osr2Ires-Cre1, 5'-GAA TTC GCC AAT GAC AAG ACG CTG-3'; Osr2Ires-Cre2, 5'-CTA CAA GGA TCT AGC ACA TGC TG-3'), Rosa26R (R1295, 5'-GCG AAG AGT TTG TCC TCA ACC-3'; R523, 5'-GGA GCG GGA GAA ATG GAT-3'; R26F2, 5'-AAA GTC GCT CTG AGT TGT TAT-3'). To analyze the level of Cre activity, Osr2Ires-Cre, OC-Cre mice were crossed with Rosa26R mice, and the mandibles (P0 and P8) of the double-transgenic mice were processed for X-gal staining, as described previously [18].
For histology analysis, the mice at the age of P0 to P28 were sacrificed and their heads and mandibles were carefully dissected. Tissues were fixed in 4% paraformaldehyde and decalcified in 10% ethylenediaminetetraacetic acid/phosphate buffered saline solution for 1 to 4 weeks at 4℃. The decalcified tissues were dehydrated through a graded ethanol series, embedded in paraffin, and sectioned at 5 µm thickness. Slides were stained with hematoxylin and eosin.
For immunohistochemistry, sections were treated with 3% hydrogen peroxide and incubated with rabbit polyclonal antibodies against osterix (Osx; 1:200, Abcam Inc., Cambridge, MA, USA), Dspp (1:200, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), phosphate regulating endopeptidase homology on the X chromosome (Phex; 1:50, Sigma-Aldrich, St. Louis, MO, USA), Dmp1 (1:750, Takara Bio Inc., Shiga, Japan), biglycan (Bgn; 1:800, Dr. Larry Fisher). Histostain Plus primary kit (Zymed Laboratories, San Francisco, CA, USA) was used according to the manufacturer's instructions.
The tooth germ of the mandibular at E14.5 was dissected from embryo. The explants were placed on filters supported by metal grid in a tissue culture dish and cultured for 1 day during genotyping. The host mouse was anesthetized using pentobarbital (0.5 mg/10 g body weight) and the explants were grafted under the kidney capsule according to standard procedure. Two weeks after transplantation, the host mice were sacrificed and the grafts were processed for histological analysis.
The Osr2Ires-Cre and OC-Cre transgene directed their specific activity of Cre recombinase at dental mesenchyme and odontoblasts respectively, as β-galactosidase activity was observed in the dental mesenchyme of Osr2CreR26R double transgenic mouse at E14.5 (Fig. 1A) and in odontoblasts of mandibular molars in OCCreR26R double transgenic mouse at P8 (Fig. 1B, C). Based on these results, we crossed Osr2Ires-Cre transgenic mice with Smad4fl/fl mice for targeting Smad4 ablation at the stage of initial coronal dentin formation. OC-Cre transgenic mice were used for targeting the stage of initial root dentin formation and long term observation of coronal dentin formation.
Since odontoblast differentiation was impaired following the disruption of Smad4 in dental mesenchyme, histological differences in odontoblast were compared with the mandibular molar of Osr2CreSmad4 and control mice (Fig. 2A, D). At newborn stage, Osr2CreSmad4 mice have a layer of non-polarized cuboidal cells with centrally located nucleus during coronal dentin formation while control mice have polarized odontoblasts. In addition, dentin matrix was absent in Osr2CreSmad4 mice (Fig. 2D). Immunohistochemistry of mandibular molars revealed that lower or no Osx and Phex were expressed in the odontoblasts of Osr2CreSmad4 mice (Fig. 2E, F) while highly expressed in differentiating odontoblast and odontoblastic processes in control mice (Fig. 2B, C). Osr2CreSmad4 mice die within a day after birth, precluding investigation of tooth development at later stage. To determine whether the abnormal odontoblast differentiation in Osr2CreSmad4 is due to delayed development, we performed kidney capsule transplantation. Mandibular first molars of embryo at E14.5 were collected and cultured for one day. By following the genotype results, it was transplanted on the kidney capsule of wild type mice and observed after 2 weeks (Fig. 2G–L). Interestingly, the tooth development of Osr2CreSmad4 mice was delayed even with normal enamel formation with well differentiated ameloblasts. However, enamel and dentin were formed with well differentiated ameloblasts and odontoblasts in control mice. Interestingly, ectopic hard tissue with cellular inclusion was formed by odontoblasts with a distinct difference from former produced dentin Osr2CreSmad4 mice (Fig. 2G, J). Cellular inclusion was commonly detected in ectopic hard tissue at cervical region of root dentin in the OCCreSmad4 mice targeting at the stage of initial root dentin formation (Fig. 2M, N). Immunohistochemistry after kidney capsule transplantation revealed that Osx was highly expressed in differentiating polarized odontoblasts in control mice while expressed in matrix forming cells and the cells trapped in dentin matrix in Osr2CreSmad4 mice (Fig. 2H, K). However, the expression of Phex was obviously decreased in the odontoblast of Osr2CreSmad4 mice when compared to control mice after kidney capsule transplantation (Fig. 2I, L). These results indicated that inactivation of TGF-β/BMP signaling in odontoblasts may disturb the differentiation processes of odontoblasts during tooth development.
Ablation of Smad4 in odontoblasts was established in OCCreSmad4 mice as seen the specific activity of Cre activity in odontoblasts of mandibular molars by OCCreR26R double transgenic mouse (Fig. 1). Histological analysis of mandibular molars revealed that dentin thickness was definitely decreased in OCCreSmad4 mice compared to control mice. In addition, the odontoblasts have shorter height and flattened morphology in OCCreSmad4 mice than in control mice, implying the loss of odontoblast polarity by ablation of Smad4 in odontoblasts. At the age of P10, coronal dentin thickness did not show any remarkable difference between control and OCCreSmad4 mice (Fig. 3A, E, I). Interestingly, coronal dentin thickness was slightly increased in the OCCreSmad4 mice while dramatically increased in control mice at the age of P14, P21, and P28 (Fig. 3B–D, F–H). These results indicate that inactivation of TGF-β/BMP signaling in odontoblasts may disturb dentin matrix production during dentin formation.
To determine molecular changes in odontoblasts and dentin formation following ablation of Smad4, immunohistochemical staining was performed with mandibular tissue sections of control and OCCreSmad4 mice at P10. Dspp was slightly expressed in odontoblasts and dentinal tubules in OCCreSmad4 mice while highly expressed in control mice (Fig. 4A, D). Dmp1 was observed in dentin matrix of OCCreSmad4 mice with strong expression while weak expression in control mice (Fig. 4B, E). Bgn, a proteoglycan, exhibited specific expression at pre-dentin in both control and OCCreSmad4 mice (Fig. 4C, F).
In this study, we investigated the significance of Smad4, a key intracellular signaling mediator of TGF-β/BMP signaling, by tissue-specific ablation under the control of Osr2 or OC promoter during tooth development. We found that during dentinogenesis the ablation of Smad4 with two types of gene targeting by different Cre activation commonly results in disturbed differentiation of odontoblasts and abnormal bone-like dentin formation, which observed at the stage of initial coronal dentin formation as well as initial root dentin formation.
TGF-β superfamily is comprised of many members, such as TGF-βs, Nodal, Activin, and BMPs. TGF-β/BMP signaling transmits initial signals across the plasma membrane through the formation of heteromeric complexes of specific type I and type II serine/threonine kinase receptors. The type I receptor is phosphorylated and then activates specific type II receptor. Activated type I receptors initiate intracellular signaling through phosphorylation of specific Smad proteins, R-Smads. Activated R-Smads form a complex with Co-Smad and Smad4 and then translocate into the nucleus to direct transcriptional response [19]. TGF-β/BMP signaling plays an important role in regulating extensive processes including cell proliferation, differentiation, apoptosis, migration, and extracellular matrix remodeling [620]. From long ago, TGF-β/BMP signaling has been implicated to have an important role during dentin formation as seen that cranial neural crest cell–derived odontoblasts secret pre-dentin and dentin following terminal differentiation [21]. Odontoblasts are differentiated from the dental mesenchymal cells in dental papilla under the influence of the inner dental epithelium. Odontoblasts become tall and in columnar shape with differentiation [21]. Smad4, the intracellular mediator for the TGF-β/BMP signaling, plays important role in regulating early tooth development [22]. Among the TGF-β superfamily, TGFβ-1, TGFβ-2, TGFβ-3, BMP2, BMP4, BMP7, and follistatin are expressed in the inner enamel epithelium, dental papilla and in polarizing and functional odontoblasts. Exogenous TGFβ-1, BMP2, BMP4, and BMP7 can induce odontoblast differentiation and dentin formation in dental papilla cells in vitro [23242526]. These data indicate that TGF-β/BMP signaling play critical roles in odontoblast differentiation and dentin formation. In our data, Osr2CreSmad4 mice exhibited disturbed differentiation of odontoblasts and abnormal bone-like structures instead of dentin formation. The results suggest that conditional inactivation of Smad4 cause morphological and functional deficiency in odontoblasts during odontoblast differentiation and dentin formation, implying that Smad4-dependent TGF-β/BMP signaling plays a significant role in odontoblast differentiation and dentin formation during tooth development. In addition, impaired dentin formation was commonly exhibited in different types of Smad4 ablation models such as Dspp-Cre;Smad4, OC-Cre;Smad4, and Col-Cre;Smad4 at the crown, cervix, and root furcation area of molars respectively [27]. These abnormalities suggest the requirement of Smad4-dependent TGF-β/BMP signaling for appropriate dentin formation. Also, exogenous TGF-β1 regulates Dspp and Dmp1 expression in odontoblast cell lines [2829], indicating that TGF-β/BMP signaling regulates dentin matrix secretion. In our results, OCCreSmad4 mice exhibited thinner crown dentin and bone-like root dentin structures in the cervical region when compared to control mice. This abnormality suggests the failure of appropriate odontoblast differentiation and resultant reduction of dentin matrix apposition.
Taken together, our results suggest that Smad4-dependent TGF-β/BMP signaling plays a critical role in odontoblast differentiation and dentin formation during tooth development.
Figures and Tables
Fig. 1
Localization of Cre recombinase for conditional inactivation of Smad4 in dental mesenchyme. β-Galactosidase activities are shown in the dental mesenchyme of Osr2CreR26R at embryo 14.5 (E14.5) (A) and in coronal odontoblasts of OCCreR26R mice at postnatal 8 (P8) (B), respectively. (C) Enlarged white-boxed area in panel B is shown. d, dentin; DM, dental mesenchyme; Od, odontoblasts; OE, oral epithelium. Scale bars=100 µm (A), 200 µm (B), 25 µm (C).

Fig. 2
Impairment of odontoblast differentiation and dentin formation by Smad4 inactivation during dentinogenesis. H&E staining of lower molar tooth germs of control and Osr2CreSmad4 mice at newborn stage (A, D). Immunohistochemical staining for Osx (B, E), Phex (C, F) with mandibular molar tooth germs of control and Osr2CreSmad4 mice at postnatal 0 (P0). Osx and Phex were highly expressed in coronal odontoblasts in control mice (B, C) while lower expression of Osx and Phex were detected in mesenchymal cells (black arrows) in Osr2CreSmad4 mice (E, F). Root forming area of mandibular molar tooth germs from control and Osr2CreSmad4 mice after kidney capsule transplantation was histologically analyzed at embryo (E) 14.5+1+2 wk (G–L). Immunohistochemical staining for Osx (H, K), Phex (I, L) with mandibular molar tooth germs of control and Osr2CreSmad4 mice. The same area of mandibular molar tooth germs from control and OCCreSmad4 mice at P21 was analyzed by H&E staining (M, N). Osx and Phex were highly expressed in differentiating odontoblasts at cervical dentin in control mice (H, I) while the lower expression of Osx was observed in entrapped cells in a bone-like tissue of Osr2CreSmad4 mice (K). The expression of Phex were not detected in the entrapped cells in Osr2CreSmad4 mice (L). The asterisks in panels J and N indicate bone-like structure of ectopic hard tissue formation. Am, ameloblasts; H&E, hematoxylin and eosin; Od, odontoblasts; Osx, osterix; Phex, phosphate regulating endopeptidase homology on the X chromosome. Scale bar=50 µm.
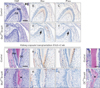
Fig. 3
Reduced dentin matrix apposition in OCCreSmad4 mice. Hematoxylin and eosin staining of crown dentin of control (A–D) and OCCreSmad4 mice (E–H) at postnal (P) 10, P14, P21, and P28. Dentin thickness was compared after the measurement of crown dentin thickness with control and OCCreSmad4 mice at P10, P14, P21, and P28 (n=5, each genotype for stage, respectively) (I). d, dentin; Od, odontoblasts. **P<0.01. Scale bar=50 µm.
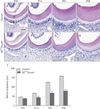
Fig. 4
Molecular changes in odontoblasts and dentin matrix with disruption of Smad4 in odontoblasts. Immunohistochemical staining of mandibular molar crown dentin in control (A–C) and OCCreSmad4 mice (D–F) at P10. Immunohistochemical staining for Dspp (A, D), Dmp1 (B, E), and Bgn (C, F) with mandibular molar of control and OCCreSmad4 mice. The expression of Dspp was detected in odontoblast and dentinal tubules in control mice (A) while broad expression in pre-dentin and pulp of OCCreSmad4 mice (D). The expression of Dmp1 was increased in OCCreSmad4 mice (E) compared to control (B). The expression of Bgn was not changed (C, F). Bgn, biglycan; d, dentin; Dmp1, dentin matrix protein 1; Dspp, dentin sialophosphoprotein; Od, odontoblasts. Scale bar=50 µm.
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (No. 2011-0027790 and 2014R1A1A2056700).
References
1. Thesleff I. Epithelial-mesenchymal signalling regulating tooth morphogenesis. J Cell Sci. 2003; 116(Pt 9):1647–1648.
2. Ruch JV, Lesot H, Bègue-Kirn C. Odontoblast differentiation. Int J Dev Biol. 1995; 39:51–68.
3. Lesot H, Lisi S, Peterkova R, Peterka M, Mitolo V, Ruch JV. Epigenetic signals during odontoblast differentiation. Adv Dent Res. 2001; 15:8–13.
4. Qin C, Baba O, Butler WT. Post-translational modifications of sibling proteins and their roles in osteogenesis and dentinogenesis. Crit Rev Oral Biol Med. 2004; 15:126–136.
5. Thesleff I, Mikkola M. The role of growth factors in tooth development. Int Rev Cytol. 2002; 217:93–135.
6. Massagué J. How cells read TGF-beta signals. Nat Rev Mol Cell Biol. 2000; 1:169–178.
7. Oka S, Oka K, Xu X, Sasaki T, Bringas P Jr, Chai Y. Cell autonomous requirement for TGF-beta signaling during odontoblast differentiation and dentin matrix formation. Mech Dev. 2007; 124:409–415.
8. Rakian A, Yang WC, Gluhak-Heinrich J, Cui Y, Harris MA, Villarreal D, Feng JQ, Macdougall M, Harris SE. Bone morphogenetic protein-2 gene controls tooth root development in coordination with formation of the periodontium. Int J Oral Sci. 2013; 5:75–84.
9. Gluhak-Heinrich J, Guo D, Yang W, Harris MA, Lichtler A, Kream B, Zhang J, Feng JQ, Smith LC, Dechow P, Harris SE. New roles and mechanism of action of BMP4 in postnatal tooth cytodifferentiation. Bone. 2010; 46:1533–1545.
10. Lan Y, Jiang R. Sonic hedgehog signaling regulates reciprocal epithelial-mesenchymal interactions controlling palatal outgrowth. Development. 2009; 136:1387–1396.
11. Wall NA, Hogan BL. TGF-beta related genes in development. Curr Opin Genet Dev. 1994; 4:517–522.
12. Xu X, Jeong L, Han J, Ito Y, Bringas P Jr, Chai Y. Developmental expression of Smad1-7 suggests critical function of TGF-beta/BMP signaling in regulating epithelial-mesenchymal interaction during tooth morphogenesis. Int J Dev Biol. 2003; 47:31–39.
13. Metzger D, Chambon P. Site- and time-specific gene targeting in the mouse. Methods. 2001; 24:71–80.
14. Yang X, Li C, Herrera PL, Deng CX. Generation of Smad4/Dpc4 conditional knockout mice. Genesis. 2002; 32:80–81.
15. Lan Y, Wang Q, Ovitt CE, Jiang R. A unique mouse strain expressing Cre recombinase for tissue-specific analysis of gene function in palate and kidney development. Genesis. 2007; 45:618–624.
16. Tan X, Weng T, Zhang J, Wang J, Li W, Wan H, Lan Y, Cheng X, Hou N, Liu H, Ding J, Lin F, Yang R, Gao X, Chen D, Yang X. Smad4 is required for maintaining normal murine postnatal bone homeostasis. J Cell Sci. 2007; 120(Pt 13):2162–2170.
17. Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999; 21:70–71.
18. Kim TH, Lee JY, Baek JA, Lee JC, Yang X, Taketo MM, Jiang R, Cho ES. Constitutive stabilization of ss-catenin in the dental mesenchyme leads to excessive dentin and cementum formation. Biochem Biophys Res Commun. 2011; 412:549–555.
19. Chen G, Deng C, Li YP. TGF-beta and BMP signaling in osteoblast differentiation and bone formation. Int J Biol Sci. 2012; 8:272–288.
20. Chai Y, Slavkin HC. Prospects for tooth regeneration in the 21st century: a perspective. Microsc Res Tech. 2003; 60:469–479.
21. Ruch JV. Patterned distribution of differentiating dental cells: facts and hypotheses. J Biol Buccale. 1990; 18:91–98.
22. Ko SO, Chung IH, Xu X, Oka S, Zhao H, Cho ES, Deng C, Chai Y. Smad4 is required to regulate the fate of cranial neural crest cells. Dev Biol. 2007; 312:435–447.
23. Bègue-Kirn C, Smith AJ, Ruch JV, Wozney JM, Purchio A, Hartmann D, Lesot H. Effects of dentin proteins, transforming growth factor beta 1 (TGF beta 1) and bone morphogenetic protein 2 (BMP2) on the differentiation of odontoblast in vitro. Int J Dev Biol. 1992; 36:491–503.
24. Nakashima M. Induction of dentin formation on canine amputated pulp by recombinant human bone morphogenetic proteins (BMP)-2 and -4. J Dent Res. 1994; 73:1515–1522.
25. Sloan AJ, Rutherford RB, Smith AJ. Stimulation of the rat dentine-pulp complex by bone morphogenetic protein-7 in vitro. Arch Oral Biol. 2000; 45:173–177.
26. Unda FJ, Martin A, Hilario E, Bègue-Kirn C, Ruch JV, Aréchaga J. Dissection of the odontoblast differentiation process in vitro by a combination of FGF1, FGF2, and TGFbeta1. Dev Dyn. 2000; 218:480–489.
27. Kim TH, Bae CH, Lee JY, Lee JC, Ko SO, Chai Y, Cho ES. Temporo-spatial requirement of Smad4 in dentin formation. Biochem Biophys Res Commun. 2015; 459:706–712.
28. He WX, Niu ZY, Zhao SL, Jin WL, Gao J, Smith AJ. TGF-beta activated Smad signalling leads to a Smad3-mediated down-regulation of DSPP in an odontoblast cell line. Arch Oral Biol. 2004; 49:911–918.
29. Unterbrink A, O'Sullivan M, Chen S, MacDougall M. TGF beta-1 downregulates DMP-1 and DSPP in odontoblasts. Connect Tissue Res. 2002; 43:354–358.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download