Abstract
Sex determination is the preliminary step in every forensic investigation and the hard palate assumes significance in cranial sexing in cases involving burns and explosions due to its resistant nature and secluded location. This study analyzes the sexing potential of incisive foramen to posterior nasal spine length, palatine process of maxilla length, horizontal plate of palatine bone length and transverse length between the greater palatine foramina. The study deviates from the conventional method of measuring the maxillo-alveolar length and breadth as the dimensions considered in this study are more heat resistant and useful in situations with damaged alveolar margins. The study involves 50 male and 50 female adult dry skulls of Indian ethnic group. The dimensions measured were statistically analyzed using Student's t test, binary logistic regression and receiver operating characteristic curve. It was observed that the incisive foramen to posterior nasal spine length is a definite sex marker with sex predictability of 87.2%. The palatine process of maxilla length with 66.8% sex predictability and the horizontal plate of palatine bone length with 71.9% sex predictability cannot be relied upon as definite sex markers. The transverse length between the greater palatine foramina is statistically insignificant in sexing crania (P=0.318). Considering a significant overlap of values in both the sexes the palatal dimensions singularly cannot be relied upon for sexing. Nevertheless, considering the high sex predictability of incisive foramen to posterior nasal spine length this dimension can definitely be used to supplement other sexing evidence available to precisely conclude the cranial sex.
Sex determination of skeleton is a preliminary step in every medicolegal investigation and the cranium and the pelvis are the most reliable sex determinants [12]. It is often difficult to determine sex accurately in burns and explosions [34]. The soft tissue distortion and bony fragmentation caused by heat often impedes sex determination [5]. In a simulation study by Holland [6], the base of the cranium was exposed to temperatures equivalent to fire accidents. It was observed that the basicranial dimensions were not altered significantly despite subjugation to high temperatures. This study proves that the anatomical structures such as the hard palate located in this region are heat resistant and hence ideal for sex determination [6]. In a study by Rogers [7], 17 morphological features of the cranium were analyzed for accurate sexing. This included nasal aperture, zygomatic extension, malar size and rugosity, supra-orbital ridge, chin form, nuchal crest, mastoid size, nasal size, mandibular symphysis and ramus size, forehead shape, and hard palate [7]. Mahakkanukrauh et al. [8] have analyzed 25 cranial dimensions of which 24 exhibit statistically significant sexual dimorphism. Bony parameters such as the maximum length of humerus and its vertical head diameter exhibit a combined accuracy of 87% [9]. It is clear from these studies that providing a conclusive report on sex usually requires combined analysis of more than one sexually dimorphic parameter. Therefore, every study that defines new parameters helps in confirming sex more precisely. The hard palate is a bony structure separating the oral and nasal cavities, formed by the union of the palatine processes of maxillae and the horizontal plates of palatine bones linked by a cruciform suture [10]. There is sufficient scientific literature regarding the variability of palatal dimensions [11121314]. There are also studies on palatal rugal patterns that conclusively establish their sexing significance [1516]. Some authors have analyzed the palatal dimensions using computed tomography (CT) scans and confirmed their sexing significance [17]. Others have analyzed palatal dimensions in dry skulls where the maxillo-alveolar length and maxillo-alveolar breadth were conventionally used as sexing parameters [18]. However, we have deviated from the conventional method by measuring the length from the posterior margin of the incisive foramen to the tip of the posterior nasal spine. This dimension was statistically analyzed and was found to have 87.2% sex predictability. The reason for deviating from the traditional method is that in high amplitude fire accidents and explosions this dimension is always preserved as it lies in the midline. Moreover, studies also show that the bone density of palate is maximum in the incisive canal zone and in the median area behind the incisive foramen [19]. Further, this dimension remains unaffected by damage to the alveolar margins as they are excluded from measurement. The study also analyzes the sex predictability of palatine process of maxilla and horizontal plate of palatine bone as in some situations only bone fragments are available for forensic analysis.
This study involving 100 adult dry skulls was conducted in the Department of Anatomy in one of the medical institutes in India. Only adult skulls that were completely ossified without any skeletal deformities were included in the study. Skulls with palatal defects were excluded. All skulls were procured from Urva cemetery, Ladyhill, Mangaluru city, Karnataka state, India. The 100 skulls included 50 male skulls and 50 female skulls aged between 26 to 65 years. All palatal dimensions were measured using a sliding digital calliper (Lianying 0005, Zhejiang, China) graduated to the last 0.01 mm.
Specific landmarks were first defined on the hard palate. When the palatine processes of the maxillae and the horizontal plates of palatine bones meet at a single point, as shown in Fig. 1, that point was defined as 'common meeting point' (CMP). When the palatine processes of the maxillae and the horizontal plates of palatine bones do not meet at a single point, as shown in Fig. 2, the anterior meeting point and the posterior meeting point were defined.
When all the four palatine bones meet at a common point, the sagittal dimensions were measured as shown in Figs. 3, 4, 5. The incisive foramen to posterior nasal spine length was measured as shown in Fig. 3. This measurement was taken from the posterior end of the incisive foramen to the tip of the posterior nasal spine. The palatine process of maxilla length was measured as shown in Fig. 4. This measurement was taken from the posterior end of the incisive foramen to the CMP. The horizontal plate palatine bone length was measured as shown in Fig. 5. This measurement was taken from the CMP to the tip of the posterior nasal spine.
When all the four palatine bones do not meet at a common point, the sagittal dimensions were measured as shown in Figs. 6, 7, 8. The incisive foramen to posterior nasal spine length was measured as shown in Fig. 6. This measurement was taken from the posterior end of the incisive foramen to the tip of the posterior nasal spine. The palatine process of maxilla length was measured as shown in Fig. 7. This measurement was taken from the posterior end of the incisive foramen to the anterior meeting point. The horizontal plate palatine bone length was measured as shown in Fig. 8. This measurement was taken from the anterior meeting point to the tip of the posterior nasal spine.
The transverse length between the greater palatine foramina was measured as shown in Fig. 9. This measurement was taken from the inner margin of right greater palatine foramen to the inner margin of the left greater palatine foramen.
All the dimensions were measured by a single observer to prevent inter-observer errors. All measurements were repeated twice by the observer and the results were compared. If there was a difference of more than 0.1 mm then a third measurement was taken [20]. Intra-examiner reliability was evaluated by re-measuring 31 skulls after ten days.
The dimensions measured were statistically analysed using SPSS version 20.0 (IBM Co., Armonk, NY, USA), two tailed Student's t test (P<0.05), linear correlation, binary logistic regression and receiver operating characteristic curve. Paired t test and Dahlberg's index was used to check systematic and casual error and the intra-examiner reliability. Binary logistic regression was applied to obtain an equation that determines the sex of the individual. An equation was obtained for each variable measured and on applying the equation to the variable value a predicted value was obtained. In this equation, the cut off value was 0.5 and hence if the predicted value was equal to or more than 0.5, it was considered female and less than 0.5, it was considered to be male. The predicted probabilities of binary logistic regression were analyzed using receiver operating characteristic curve. The receiver operating characteristic curve is an indicator of the equation' s ability to distinguish two groups and the area under the curve measures the strength of the equation. If the area is less than 0.5, it indicates that any observation is a matter of chance and a value close to 1 indicates that the equation strongly discriminates two groups.
The mean incisive foramen to posterior nasal spine length was greater than the mean transverse length between the greater palatine foramina with a P-value of <0.001, and by conventional criteria this difference is considered extremely statistically significant and is also consistent with the shape of the hard palate. The descriptive statistics of all the dimensions and their significance is summarized in Table 1. It is evident from the table that all the dimensions except the transverse length between the greater palatine foramina are statistically significant in sexing the crania. The incisive foramen to posterior nasal spine length has a P-value less than 0.001 and hence is extremely significant in sex determination. The palatine process of maxilla length and the horizontal plate palatine bone length have a P-value of 0.004 and a P-value less than 0.001, respectively. Both the values are less than 0.05 and hence statistically significant. The transverse length between the greater palatine foramina has a P-value of 0.318 and hence is statistically insignificant. This implies that all the three anteroposterior dimensions of the hard palate are sexually dimorphic but the transverse dimension between the greater palatine foramina does not exhibit sexual dimorphism.
Binary logistic regression analysis was used to obtain an equation that can determine the sex of the individual. The regression equations obtained for each of these significant variables and their significance is depicted in Table 2. It was observed that all the equations have a P-value less than 0.05 and hence are statistically significant.
The sex predictability percentage of individual dimensions was calculated using receiver operating characteristic curve. The area under the curve is a measure of strength of the equation and hence a measure of sex predictability of the variable. The predicted probabilities of binary logistic regression were analyzed using this curve. The area under the curve was 0.872 for incisive foramen to posterior nasal spine length, 0.668 for palatine process of maxilla length, and 0.719 for horizontal plate palatine bone length. All the values of area are more than 0.5 which suggest that the variables significantly discriminate the two groups which in this case are males and females.
As observed the incisive foramen to posterior nasal spine length with sex predictability of 87.2% is a definite sex marker. However, the palatine process of maxilla length with 66.8% sex predictability and the horizontal plate of palatine bone length with 71.9% sex predictability cannot be relied upon as definite sex markers. The receiver operating characteristic curve for incisive foramen to posterior nasal spine length is shown in Fig. 10, for palatine process of maxilla length (Fig. 11) and for horizontal plate palatine bone length (Fig. 12).
No linear correlation was observed between the sex-pooled incisive foramen to posterior nasal spine length and the transverse length between the greater palatine foramina (Pearson correlation value of –0.118 and an insignificant P-value of 0.244).
Forensic anthropology is a branch of science, which applies scientific information regarding sexually dimorphic traits exhibited by the human skeleton for administration of law and justice [21]. Human cranial and pelvic components exhibit significant sexual dimorphism and hence, are considered to be foremost in predicting skeletal sex with high accuracy [22]. The mastoid triangle, foramen magnum, hard palate, zygomatic arch, the pterion position, supra-orbital ridges, forehead slope and the orbital margins, are only a few of the several sexually dimorphic parameters, validated in the cranium by researchers across the globe in diverse populations [23242526]. The significance of hard palate in cranial sexing is attributed to its resistant nature and secluded anatomical position in the base of the skull. Hence, it is often available for forensic analysis even when the surface skeletal components are damaged as in fire accidents and explosions [6]. The skeletal components forming the hard palate develop by membranous ossification [27]. Despite membranous development the hard palate is highly resistant to heat [6]. Moreover, its bone density is maximum in the incisive canal zone and in the median area behind the incisive foramen [19]. Hence, in this study we have statistically analyzed this region of maximum bone density of the hard palate extending from the incisive canal to the posterior nasal spine. This dimension was found to have 87.2% sex predictability which is considered significant for forensic anthropology.
The conventional method of studying the palate in a dry adult skull involves measurement of maxillo-alveolar length and maxillo-alveolar breadth [18]. Here, two bony landmarks namely the prosthion and the alveolon are defined. The prosthion is the anterior point on the alveolar border of maxilla in the mid-sagittal plane between the two central incisors. The alveolon is the point where the mid-sagittal plane intersects a transverse plane at the posterior margin of the maxillary alveolar processes [28]. The linear distance between the prosthion and alveolon is the maxillo-alveolar length. The maximum breadth at the second maxillary molar from the outer surface of one alveolar border to the outer surface of the other is the maxillo-alveolar breadth [28]. In a study by Ramamoorthy et al. [18], the maxillo-alveolar length was found to be extremely significant in cranial sexing with a P-value of 0.001. The maxillo-alveolar breadth was found to be insignificant with a P-value of 0.103 [18]. However, in this study the sex predictability percentage or sexing accuracy of these dimensions was not determined and only their significance was ascertained. In a scenario, where the alveolar margins are pathologically deformed or fragmented due to trauma these conventional dimensions cannot be used for cranial sexing. In such situations the dimensions measured in this study assume significance as they do not involve the maxilla and the alveolar margins. The incisive foramen to posterior nasal spine length lies in the midline and is usually preserved for forensic sexing. In a study by Nishii et al. [19], in 160 cone-beam CT scans, it was observed that the palatal bone density and thickness was maximum in the incisive canal zone and in the median area behind the incisive foramen. This implies that the dimension extending from the posterior margin of incisive foramen to the tip of the posterior nasal spine is the most heat resistant part of the palate and hence is most suitable for forensic sexing.
In the present study, the incisive foramen to the posterior nasal spine length is proven to be the most reliable palatal dimension with 87.2% sexing accuracy. The palatine process of maxilla length with 66.8% sex predictability and the horizontal plate of palatine bone length with 71.9% sex predictability cannot be relied upon as definite sex markers. The transverse length between the greater palatine foramina is insignificant in sexing the crania.
In a study in 100 adult dry skulls, the incisive fossa to basion length was found to be extremely significant in cranial sexing with a P-value of 0.004. The incisive fossa to greater palatine foramina length was found to be moderately significant with a P-value less than 0.05. The transverse dimension between the greater palatine foramina was insignificant in sexing with a P-value of 0.593. Two logistic regression models were derived for sex determination. The first model based on incisive fossa to basion length, incisive fossa to right greater palatine foramen length and incisive fossa to left greater palatine foramen length determined sex with 63% accuracy. The second model based on incisive fossa to basion length determined sex with 65% accuracy [29]. In another similar study, involving 200 adult dry skulls using logistic regression and discriminate function, the incisive fossa to basion length was found to have a sexing accuracy of 79.9% [30]. From the above mentioned studies, it can be inferred that the incisive fossa to basion length has a sexing accuracy of 65% to 79.9%.
In another study involving 1,200 sinus CT scans of hard palate, it was observed that orale-spina nasalis posterior distance, the greater palatine canal depth and the anterior width of palatal arch exhibited significant sexual dimorphism. All the dimensions measured were found to be extremely significant in cranial sexing with a P-value less than 0.001. In this study, two logistic regression equations were derived. The first equation was based on orale-spina nasalis posterior distance and had a sexing accuracy of 68.35%. The second equation was based on right greater palatine canal depth, orale-spina nasalis posterior distance and the anterior width of palatal arch and had a sexing accuracy of 78.37% [17]. The orale-spina nasalis posterior distance measured in this study is marginally longer than the incisive fossa to posterior nasal spine distance measured in our study. The orale being a fixed bony landmark seen on the CT-scan is located a few millimetres anterior to incisive fossa. In our present study, the incisive fossa to posterior nasal spine distance has a sexing accuracy of 87.2% and in this study, the orale-spina nasalis posterior distance has a sexing accuracy of 68.35%.
In a three dimensional study on the hard palate and basicranium in 176 adult dry skulls, it was observed that the palate was deeper and longer and the cranial base shorter in males than females. The study concluded that if both the shape and size of palate and basicranium were combined, it was possible to determine sex with an accuracy of 90.4% for basicranium and 74.8% for hard palate [31].
From the studies mentioned above, it may be inferred, that the sagittal dimensions of the hard palate and the basicranium are extremely significant in sexing the crania. The maxilloalveolar length was extremely significant with a P-value of 0.001 [18]. The orale-spina nasalis posterior distance in CT scans was also extremely significant with a P-value less than 0.001 [17]. In our present study all the three sagittal dimensions were extremely significant; the incisive fossa to posterior nasal spine distance with P-value less than 0.001, the palatine process of maxilla length with P-value of 0.004, and the horizontal plate palatine bone length with P-value less than 0.001. The incisive fossa to basion length was also found to be extremely significant with a P-value of 0.004 [29].
Similarly, the sagittal dimensions are consistently accurate in sexing the crania. The sexing accuracy means, the accuracy of the regression model derived in the respective studies using specific dimensions that exhibited significant sexual dimorphism. The sexing accuracy is described as a percentage. The orale-spina nasalis posterior distance in CT scans showed an accuracy of 68.35% [17]. In our present study the incisive fossa to posterior nasal spine distance showed a sexing accuracy of 87.2%, the palatine process of maxilla length an accuracy of 66.8% and the horizontal plate of palatine bone length, an accuracy of 71.9%. The incisive fossa to basion length showed a sexing accuracy of 65% in one study [29] and 79.9% in another [30]. The accuracy of individual palatal dimensions in cranial sexing as observed by diverse authors is summarized in Table 3. The accuracy of combined palatal dimensions in cranial sexing as observed by diverse authors is summarized in Table 4.
We can also infer that the transverse dimensions of the palate do not exhibit sexual dimorphism. The maxilloalveolar breadth was found to be insignificant with a P-value of 0.103 [18]. The transverse distance between the greater palatine foramina was found to be insignificant in the present study with a P-value of 0.318 and in the study by Nascimento Correia Lima et al. [29] with a P-value of 0.593.
A literature defined conclusion on sexual dimorphism of hard palate dimensions can also be established based on this study and other studies preceding it. It can be stated that several dimensions of hard palate such as incisive fossa to posterior nasal spine length, incisive fossa to greater palatine foramina length, palatal size and shape, palatal depth, oralespina nasalis posterior distance, the greater palatine canal depth and the anterior width of palatal arch in CT scans, maxillo-alveolar length and incisive fossa to basion length exhibit significant sexual dimorphism.
The incisive foramen to posterior nasal spine length is a definite sex marker in cranial sexing with an accuracy of 87.2%. This dimension assumes significance in sexing crania associated with distorted alveolar margins where the maxilloalveolar length cannot be accurately measured. Previous studies have shown that the incisive canal zone and the area behind the incisive foramen are the thickest regions of the palate. Therefore, this dimension is the most resistant part of the palate and also has a secluded anatomical location and hence is usually available for forensic analysis. The palatine process of maxilla length with 66.8% sex predictability and the horizontal plate of palatine bone length with 71.9% sex predictability cannot be relied upon as definite sex markers. The transverse length between the greater palatine foramina is statistically insignificant in sexing crania. Considering a significant overlap of values in both the sexes the palatal dimensions singularly cannot be relied upon for sexing. Nevertheless, considering the high sex predictability of incisive foramen to posterior nasal spine length this dimension can definitely be used to supplement other sexing evidence available to precisely conclude the skeletal sex.
Figures and Tables
Fig. 1
The sutural morphology when all the palatine bones meet at a common point. CMP, common meeting point.
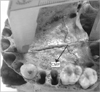
Fig. 2
The sutural morphology when the palatine bones do not meet at a common point. AMP, anterior meeting point; PMP, posterior meeting point.
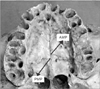
Fig. 3
The incisive foramen (IF) to posterior nasal spine (PNS) length when the bones meet at a point. CMP, common meeting point.

Fig. 4
The palatine process of maxilla length when the bones meet at a point. CMP, common meeting point; IF, incisive foramen.

Fig. 5
The horizontal plate palatine bone length when the bones meet at a point. CMP, common meeting point; PNS, posterior nasal spine.
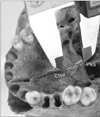
Fig. 6
Incisive foramen to posterior nasal spine (PNS) length when bones do not meet at a point. IF, incisive foramen.

Fig. 7
Palatine process of maxilla length when the bones do not meet at a common point. AMP, anterior meeting point; IF, incisive foramen.

Fig. 8
Horizontal plate palatine bone length when the bones do not meet at a point. AMP, anterior meeting point; PNS, posterior nasal spine.

Fig. 9
Transverse length between the greater palatine foramina measured in the study. LGPF, left greater palatine foramen; RGPF, right greater palatine foramen.
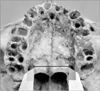
Fig. 10
The receiver operating characteristic (ROC) curve for incisive foramen to posterior nasal spine length.

Fig. 12
The receiver operating characteristic (ROC) curve for horizontal plate palatine bone length.

Table 1
Descriptive statistics of all the dimensions and their significance

Table 2
Regression equations obtained by BLR and their significance

Table 3
Sexing accuracy of individual palatal dimensions as observed by diverse authors

| Reference | Dimension measured | Sexing accuracy (%) |
|---|---|---|
| Francesquini Júnior et al. (2007) [30] | Incisive fossa to basion length | 79.9 |
| Nascimento Correia Lima et al. (2012) [29] | Incisive fossa to basion length | 65 |
| Tomaszewska et al. (2014) [17] | Orale-spina nasalis posterior distance | 68.35 |
| Kamath et al. (2015) [24] | Incisive fossa to posterior nasal spine length | 87.2 |
| Present study | Palatine process of maxilla length | 66.8 |
| Horizontal plate of palatine bone length | 71.9 |
Table 4
Sexing accuracy of combined palatal dimensions as observed by diverse authors

| Reference | Dimension measured | Combined accuracy (%) |
|---|---|---|
| Nascimento Correia Lima et al. (2012) [29] | Incisive fossa to basion length | 63 |
| Incisive fossa to right greater palatine foramen length | ||
| Incisive fossa to left greater palatine foramen length | ||
| Chovalopoulou et al. (2013) [31] | Size and shape of palate | 74.8 |
| Tomaszewska et al. (2014) [17] | Right greater palatine canal depth | 78.37 |
| Orale-spina nasalis posterior distance | ||
| Anterior width of palatal arch |
References
1. Durić M, Rakocević Z, Donić D. The reliability of sex determination of skeletons from forensic context in the Balkans. Forensic Sci Int. 2005; 147:159–164.
2. Işcan MY. Forensic anthropology of sex and body size. Forensic Sci Int. 2005; 147:107–112.
3. Patil KR, Mody RN. Determination of sex by discriminant function analysis and stature by regression analysis: a lateral cephalometric study. Forensic Sci Int. 2005; 147:175–180.
4. Graw M, Wahl J, Ahlbrecht M. Course of the meatus acusticus internus as criterion for sex differentiation. Forensic Sci Int. 2005; 147:113–117.
5. Goncalves D, Thompson TJ, Cunha E. Osteometric sex determination of burned human skeletal remains. J Forensic Leg Med. 2013; 20:906–911.
6. Holland TD. Use of the cranial base in the identification of fire victims. J Forensic Sci. 1989; 34:458–460.
7. Rogers TL. Determining the sex of human remains through cranial morphology. J Forensic Sci. 2005; 50:493–500.
8. Mahakkanukrauh P, Sinthubua A, Prasitwattanaseree S, Ruengdit S, Singsuwan P, Praneatpolgrang S, Duangto P. Craniometric study for sex determination in a Thai population. Anat Cell Biol. 2015; 48:275–283.
9. Lee JH, Kim YS, Lee UY, Park DK, Jeong YG, Lee NS, Han SY, Kim KY, Han SH. Sex determination using upper limb bones in Korean populations. Anat Cell Biol. 2014; 47:196–201.
10. Williams PL, Bannister LH, Berry MM, Collins P, Dyson M, Dussek JE, Ferguson MW. Gray's anatomy. 38th ed. London: Churchill Livingstone;1995.
11. Yemitan TA, Dacosta OO, Sanu OO, Utomi IL. Metric analysis of the hard palate in children with digit sucking habits. Odontostomatol Trop. 2013; 36:11–16.
12. Rizell S, Barrenäs ML, Andlin-Sobocki A, Stecksén-Blicks C, Kjellberg H. Palatal height and dental arch dimensions in Turner syndrome karyotypes. Eur J Orthod. 2013; 35:841–847.
13. Berwig LC, Silva AM, Côrrea EC, Moraes AB, Montenegro MM, Ritzel RA. Hard palate dimensions in nasal and mouth breathers from different etiologies. J Soc Bras Fonoaudiol. 2011; 23:308–314.
14. Jung BA, Wehrbein H, Heuser L, Kunkel M. Vertical palatal bone dimensions on lateral cephalometry and cone-beam computed tomography: implications for palatal implant placement. Clin Oral Implants Res. 2011; 22:664–668.
15. Kotrashetti VS, Hollikatti K, Mallapur MD, Hallikeremath SR, Kale AD. Determination of palatal rugae patterns among two ethnic populations of India by logistic regression analysis. J Forensic Leg Med. 2011; 18:360–365.
16. Saraf A, Bedia S, Indurkar A, Degwekar S, Bhowate R. Rugae patterns as an adjunct to sex differentiation in forensic identification. J Forensic Odontostomatol. 2011; 29:14–19.
17. Tomaszewska IM, Fraczek P, Gomulska M, Pliczko M, Sliwińska A, Sałapa K, Chrzan R, Kowalski P, Nowakowski M, Walocha JA. Sex determination based on the analysis of a contemporary Polish population's palatine bones: a computed tomography study of 1,200 patients. Folia Morphol (Warsz). 2014; 73:462–468.
18. Ramamoorthy B, Pai MM, Prabhu LV, Muralimanju BV, Rai R. Assessment of craniometric traits in South Indian dry skulls for sex determination. J Forensic Leg Med. 2016; 37:8–14.
19. Nishii Y, Sameshima GT, Mah JK, Enciso R, Takaki T, Sueishi K. Hard palate thickness for temporary anchorage devices placement: differences in sex and ethnicity. Orthod Waves. 2014; 73:121–129.
20. Krag MH, Weaver DL, Beynnon BD, Haugh LD. Morphometry of the thoracic and lumbar spine related to transpedicular screw placement for surgical spinal fixation. Spine (Phila Pa 1976). 1988; 13:27–32.
21. da Silva RH, de Oliveira RN. Forensic anthropology and molecular biology: independent or complementary sciences in forensic dentistry? An overview. Braz J Oral Sci. 2008; 7:1575–1579.
22. Steyn M, Işcan MY. Sexual dimorphism in the crania and mandibles of South African whites. Forensic Sci Int. 1998; 98:9–16.
23. Suazo GI, Zavando MD, Smith RL. Sex determination using mastoid process measurements in Brazilian skulls. Int J Morphol. 2008; 26:941–944.
24. Kamath VG, Asif M, Shetty R, Avadhani R. Binary logistic regression analysis of foramen magnum dimensions for sex determination. Anat Res Int. 2015; 2015:459428.
25. Monticelli F, Graw M. Investigation on the reliability of determining sex from the human os zygomaticum. Forensic Sci Med Pathol. 2008; 4:181–186.
26. Bigoni L, Velemínská J, Brůzek J. Three-dimensional geometric morphometric analysis of cranio-facial sexual dimorphism in a Central European sample of known sex. Homo. 2010; 61:16–32.
27. Nemzek WR, Brodie HA, Hecht ST, Chong BW, Babcook CJ, Seibert JA. MR, CT, and plain film imaging of the developing skull base in fetal specimens. AJNR Am J Neuroradiol. 2000; 21:1699–1706.
28. Moore Jansen PM, Ousley SD, Jantz RL. Data collection procedures for forensic skeletal material. Knoxville, TN: Department of Anthropology, University of Tennessee;1994. p. 45–51.
29. Nascimento Correia Lima N, Fortes de Oliveira O, Sassi C, Picapedra A, Francesquini L Jr, Daruge E Jr. Sex determination by linear measurements of palatal bones and skull base. J Forensic Odontostomatol. 2012; 30:38–44.
30. Francesquini Júnior L, Francesquini MA, De La Cruz BM, Pereira SD, Ambrosano GM, Barbosa CM, Daruge Júnior E, Del Bel Cury AA, Daruge E. Identification of sex using cranial base measurements. J Forensic Odontostomatol. 2007; 25:7–11.
31. Chovalopoulou ME, Valakos ED, Manolis SK. Sex determination by three-dimensional geometric morphometrics of the palate and cranial base. Anthropol Anz. 2013; 70:407–425.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download