Abstract
The aim of this study was to provide accurate anatomical descriptions of the overall anatomy of the superior thyroid artery (STA), its relationship to other structures, and its driving patterns. Detailed dissection was performed on thirty specimens of adult's cadaveric neck specimens and each dissected specimen was carefully measured the following patterns and distances using digital and ruler. The superior thyroid, lingual, and facial arteries arise independently from the external carotid artery (ECA), but can also arise together, as the thyrolingual or linguofacial trunk. We observed that 83.3% of STAs arose independently from the major artery, while 16.7% of the cases arose from thyrolingual or linguofacial trunk. We also measured the distance of STA from its major artery. The origin of the STA from the ECA was 0.9±0.4 mm below the hyoid bone. The STA was 4.4±0.5 mm distal to the midline at the level of the laryngeal prominence and 3.1±0.6 mm distal to the midline at the level of the inferior border of thyroid cartilage. The distance between STA and the midline was similar at the level of the hyoid bone and the thyroid cartilage. Also, when the STA is near the inferior border of the thyroid cartilage, it travels at a steep angle to the midline. This latter point may be particularly important in thyroidectomies. We hope that anatomical information provided here will enhance the success of, and minimize complications in, surgeries that involve STA.
Successful surgical outcomes require superb knowledge of normal anatomy. Beyond that, the surgeon must also understand the possible variations in anatomical structure among individuals [1]. All surgeries run the risk of complications such as air leaks, bleeding, edema, and coma, which can be fatal [12345] and therefore, risks must be assessed and minimized in all situations. Complete understanding of the anatomical structure in general and the individual patient's in particular should empower the medical profession to effectively minimize risks. Given the seriousness of uncontrolled bleeding in surgery, it is particularly important to have accurate knowledge of vascular patterns and blood vessel anatomy. To this end, we have made a detailed study of the anatomy and anatomical variations of the superior thyroid artery (STA). It is our hope that this information will enable surgeons to perform surgeries that involve the STA with greater confidence of a successful outcome.
The STA arises from the external carotid artery (ECA) just below the level of the greater cornu of the hyoid bone and ends in the thyroid gland. It distributes twigs to the adjacent muscles, the upper larynx, and the neck region; and it distributes numerous branches to the thyroid gland and overlying skin [567]. Surgical procedures that involve the STA include radical neck dissection, cricothyroidotomy, thyroidectomy, reconstruction of an aneurysm, carotid endarterectomy, treatments for cancer, diagnostic and therapeutic catheterization, and plastic surgery [5789].
As is true of most vascular arrangements, the origin, thickness, driving patterns, and distribution patterns of the STA differ among individuals. Most of the studies of the STA have focused on the possible variations in its origin. In general, textbooks state that it arises from the ECA as a main branch, but Gupta et al. [7] reported that it can originate from the ECA, from the bifurcation of the common carotid artery (CCA), or from the CCA [810 11].
The skin, the thyroid gland, and the muscles adjacent to the thyroid contain STA branches. Bleeding from these sites can occur easily during surgery. Therefore, the driving patterns of the STA and its relationship to the anatomical structures adjacent to it may be even more important to surgeons than the origin of the STA. Ozgur et al. [5] performed an in-depth study of the STA including, in addition to its origin patterns, its distance from other arteries, thickness, and driving patterns to sub-branches. However, studies on the location of the STA relative to other structures are still needed. In this study, we have investigated the overall anatomy of the STA, its relationship to other structures, and its driving patterns.
Thirty specimens from cadavers, 20 men and 10 women, were examined. The cadavers were of Korean descent and had been embalmed after death. The mean age at time of death was 66.7 years, ranging from 58 to 80 years. The cadavers had no history of trauma or surgical procedures associated with the cervical region. Latex (Neoprene, Lot No. 307L146, Barsac, DuPont, France) with a red coloring agent (colorant universel, Templemars, Castorama, France) was injected into all the specimens through the CCA to permit observation of the topographic relationship between the course of the STA and the anterior cervical region surrounding it.
The skin and subcutaneous tissue overlying the platysma muscle were removed, exposing the platysma and sternocleidomastoid muscles. The anatomical structures around the STA were dissected in detail, using extreme care not to damage the underlying arteries. The origin of the STA was first determined. Next, micro-dissection was used to determine the driving patterns from the origin to the thyroid gland. Careful dissection was also undertaken to assess the position of the STA relative to the muscles, cartilage, and surrounding structures. The position relative to these known "landmarks" was measured with digital calipers (Mitutoyo, Kawaski City, Japan) and rulers.
The measurements were designed to provide the following information:
To determine if the STAs originated from a thyrolingual trunk, a linguofacial trunk, or independently from the ECA, CCA, or bifurcation.
To classify the origin of the STA as arising from the CCA, from the carotid bifurcation (CB), or from the ECA. We also measured its distance from the CB.
Third, we measured the distance of the STA from its main artery, either the ECA or CCA, to the thyroid gland, using associated anatomical structures such as the hyoid bone and the thyroid cartilage as references. These references are illustrated in Fig. 1. The midline on the mid-sagittal plane was used as a vertical reference. Three horizontal lines parallel to the hyoid bone, the prominence of the thyroid cartilage, or the inferior border of the thyroid cartilage, were also used as references. The measured distances are relative to these lines.
Arterial branches of the ECA include the ascending pharyngeal, superior thyroid, lingual, facial, occipital, facial posterior auricular, maxillary, and superficial temporal arteries. The superior thyroid, lingual, and facial arteries arise independently from the ECA, but can also arise together, as the thyrolingual or linguofacial trunk. We observed that 83.3% of the STAs (25/30) in our study arose independently from the major artery (ECA, CB, and carotid artery), while 16.7% of the cases (5/30) arose from the thyrolingual or linguofacial trunk (Table 1).
Although the STA is generally believed to arise from the ECA, variations of this general pattern exist. In 20.0% of the cases (6/30) in this study (Table 2), the STA arose from the ECA (referred to as type 1) 4.5±2.3 mm above the CB, with a range from 4 to 9.6 mm. In 40.0% of the cases (12/30), the STA arose from the CB (type II). In 40.0% of the cases (12/30), the STA arose from the CCA (type III) and was located 9.0±6.4 mm below the bifurcation. The range was from 3.0 to 21.4 mm. Photographs of these different patterns are presented in Fig. 2.
After the STA arises from its main branch (from ECA, CCA, and CB), it passes by the thyroid and cricoid cartilage on its way to the thyroid gland (Table 3). Fig. 1 shows the measured distances of the arteries along this course. At the level of the hyoid bone, the ECA was 4.9±0.5 mm from the midline. The origin of the STA from the ECA was 0.9±0.4 mm below the hyoid bone. The STA was 4.4±0.5 mm distal to the midline at the level of the laryngeal prominence of the thyroid cartilage and 3.1±0.6 mm distal to the midline at the level of the inferior border of the thyroid cartilage.
The STA is a consideration in many surgeries and accurate clinical anatomical knowledge of the STA is important to minimizing surgical complications. Most anatomical studies of the STA have been done by direct observation of its anatomy in the body, although there are a small number of angiographic studies [57121314]. These studies have mostly been concerned with identifying the pattern of origin of the STA or, occasionally, the thickness of its branches.
Gupta et al. [7] conducted an angiographic study of 25 STAs of cadavers and identified their pattern of origin. In their study, the STAs on the right side of the body arose from the ECA, CB, CCA, and in one case, from the internal carotid artery (ICA). The percentages of these different origins were 71.5%, 21.5%, 7%, and 7% of the STAs on the right side of the cadaver, respectively. When the STAs on the left side were considered, 72.5% of them arose from the ECA, 18.5% from the CB, and 9% from the ICA. An interesting and unusual finding in Gupta et al.'s study [7] was that, in one case only, the STA arose from the ICA. Some studies mention that the STA, as well as the occipital and ascending pharyngeal artery, can arise from ICA, although rarely [15].
Vascular anatomists are not in complete agreement about the incidence of the different origins of the STA. Ozgur et al. [5] reported that STAs arose from the ECA 25% of the time, from the CB 40% of the time, and from the CCA 35% of the time. In our study, the corresponding values were 20%, 40%, and 40%. Our observations are closely aligned with Ozgur et al. [5]—both groups report that the STA arose from the ECA in 20%–25% of the cases studied—but not with Gupta et al. [7], who reported that the STA arose from the ECA 72% of the time. The discrepancy may be due to differences in the criteria used by different researchers, or, as Toni et al. [13] suggested, there may be ethnic differences.
The STA can arise from the linguofacial trunk, from the thyrolingual trunk, or independently. The incidence of these variations was reported to be 7.5%, 2.5%, and 90%, respectively [5]. In our study, 16.7% of the STAs examined originated from vascular trunks, while 83.3% of the STAs had independent origins.
The information on the origins of the STA has been frequently studied, but this consideration may not be the most important to surgeons. The STA supplies the skin, muscles, and the thyroid region, areas prone to bleeding, so that the course of the STA through these areas may be critical information in surgeries. Ozgur et al. [5] observed, in regard to the STA, its basic aspect, distance from other arteries, thickness, and driving patterns to sub-branches, certainly of at least equal importance to the clinician as the origin of the STA. Likewise, information about the arterial anatomy in the skin should be useful during surgical procedures. Thus, in the final part of this study, we examined the driving pattern of the STA by measuring its orientation to major anatomical structures.
Fig. 1 presents a summary of the measurements, which should be valuable information in surgeries. In horizontal line parallel with hyoid bone, distance from mid-sagittal plane to ECA was 4.9±0.5 mm, and vertical distance from its point to STA was 0.9±0.4 mm. In horizontal line parallel with laryngeal prominence of thyroid cartilage, distance from mid-sagittal plane to STA was 4.4±0.5 mm. In horizontal line parallel with inferior border of thyroid cartilage, distance from mid-sagittal plane to STA was 3.1±0.6 mm. There was no significant difference in the distance from the midline to the STA in horizontal line parallel with hyoid bone and thyroid cartilage. As mentioned above, the STA is located 4.4±0.5 mm distance from the midsagittal plane to prominence of thyroid cartilage and 3.1±0.6 mm distance from the inferior border to thyroid cartilage, respectively. It vertically enters thyroid gland in inferior border of thyroid cartilage.
STA is not only major artery of thyroid gland but also feeding artery (about 80%) of thyroid turmor [16]. In addition, it was reported that peak velocity of ipsilateral STA in patient of the parathyroid adenoma was significantly increased [17]. For three main complications of thyroidectomy, there are recurrent laryngeal nerve injury, bleeding, and hypoparathyroidism, observed in around 2% of the patients, according to the surgeons' experiences [18]. In order to minimize these complications due to bleeding caused by surgical approach to remove tumors in thyroid and parathyroid glands, the locational relations between STA and thyroid gland should be clearly considered.
In conclusion, STA is main artery for neck skin and tissues, its peripheral muscles, and thyroid gland, and to understand its branching and locational relations with anatomical structure around it will play a great role to minimize complications including bleeding. This study research shows that the information about the locational relations with the artery's peripheral anatomical structures will be more useful than its branching status in case of surgical approach containing STA.
Figures and Tables
Fig. 1
Horizontal and vertical distances of the superior thyroid artery from its associated structures. CA, carotid artery; ECA, external carotid artery; ICA, internal carotid artery; STA, superior thyroid artery.
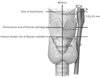
Fig. 2
Patterns of origin of the superior thyroid artery (STA). The STA can arise from the external carotid artery (type I) (A), from the carotid bifurcation (CB) (type II) (B), or from the common carotid artery (type III) (C). LP, laryngeal prominence of thyroid cartilage.

Table 1
Ramification pattern of the superior thyroid artery

| Branching pattern of superior thyroid artery | No. of specimens (%) |
|---|---|
| Commonly branched with thyrolingual or linguofacial trunk | 5 (16.7) |
| Independently branched | 25 (83.3) |
Table 2
Distance from carotid bifurcation to ramification of superior thyroid artery

Table 3
Horizontal and vertical distance from around structures
References
1. Anthony JP, Argenta P, Trabulsy PP, Lin RY, Mathes SJ. The arterial anatomy of larynx transplantation: microsurgical revascularization of the larynx. Clin Anat. 1996; 9:155–159.
2. Dedecjus M, Tazbir J, Kaurzel Z, Lewinski A, Strozyk G, Brzezinski J. Selective embolization of thyroid arteries as a preresective and palliative treatment of thyroid cancer. Endocr Relat Cancer. 2007; 14:847–852.
3. Hayashi N, Hori E, Ohtani Y, Ohtani O, Kuwayama N, Endo S. Surgical anatomy of the cervical carotid artery for carotid endarterectomy. Neurol Med Chir (Tokyo). 2005; 45:25–29.
4. Jeganath V, McElwaine JG, Stewart P. Ruptured superior thyroid artery from central vein cannulation: treatment by coil embolization. Br J Anaesth. 2001; 87:302–305.
5. Ozgur Z, Govsa F, Celik S, Ozgur T. Clinically relevant variations of the superior thyroid artery: an anatomic guide for surgical neck dissection. Surg Radiol Anat. 2009; 31:151–159.
6. Akyol MU, Koc C, Ozcan M, Ozdem C. Superior thyroid artery arising from the common carotid artery. Otolaryngol Head Neck Surg. 1997; 116(6 Pt 1):701.
7. Gupta P, Bhalla AS, Thulkar S, Kumar A, Mohanti BK, Thakar A, Sharma A. Variations in superior thyroid artery: a selective angiographic study. Indian J Radiol Imaging. 2014; 24:66–71.
8. Vazquez T, Cobiella R, Maranillo E, Valderrama FJ, McHanwell S, Parkin I, Sañudo JR. Anatomical variations of the superior thyroid and superior laryngeal arteries. Head Neck. 2009; 31:1078–1085.
9. Ozgüner G, Sulak O. Arterial supply to the thyroid gland and the relationship between the recurrent laryngeal nerve and the inferior thyroid artery in human fetal cadavers. Clin Anat. 2014; 27:1185–1192.
10. Natsis K, Raikos A, Foundos I, Noussios G, Lazaridis N, Njau SN. Superior thyroid artery origin in Caucasian Greeks: a new classification proposal and review of the literature. Clin Anat. 2011; 24:699–705.
11. Won HS, Han SH, Oh CS, Chung IH. Superior and middle thyroid arteries arising from the common carotid artery. Surg Radiol Anat. 2011; 33:645–647.
12. Banna M, Lasjaunias P. The arteries of the lingual thyroid: angiographic findings and anatomic variations. AJNR Am J Neuroradiol. 1990; 11:730–732.
13. Toni R, Della Casa C, Castorina S, Malaguti A, Mosca S, Roti E, Valenti G. A meta-analysis of superior thyroid artery variations in different human groups and their clinical implications. Ann Anat. 2004; 186:255–262.
14. Ozgur Z, Govsa F, Ozgur T. Anatomic evaluation of the carotid artery bifurcation in cadavers: implications for open and endovascular therapy. Surg Radiol Anat. 2008; 30:475–480.
15. Aggarwal NR, Krishnamoorthy T, Devasia B, Menon G, Chandrasekhar K. Variant origin of superior thyroid artery, occipital artery and ascending pharyngeal artery from a common trunk from the cervical segment of internal carotid artery. Surg Radiol Anat. 2006; 28:650–653.
16. Anagnostopoulou S, Mavridis I. Emerging patterns of the human superior thyroid artery and review of its clinical anatomy. Surg Radiol Anat. 2014; 36:33–38.
17. Varsamidis K, Varsamidou E, Mavropoulos G. Color Doppler sonography in the detection of parathyroid adenomas. Head Neck. 1999; 21:648–651.
18. Sosa JA, Bowman HM, Tielsch JM, Powe NR, Gordon TA, Udelsman R. The importance of surgeon experience for clinical and economic outcomes from thyroidectomy. Ann Surg. 1998; 228:320–330.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download