Abstract
The frontal nerve is characterized by its great content of sympathetic nerve fibers in contrast to cutaneous branches of the maxillary and mandibular nerves. However, we needed to add information about composite fibers of cutaneous branches of the nasociliary nerve. Using cadaveric specimens from 20 donated cadavers (mean age, 85), we performed immunohistochemistry of tyrosine hydroxylase (TH), neuronal nitric oxide synthase (nNOS), and vasoactive intestinal polypeptide (VIP). The nasocilliary nerve contained abundant nNOS-positive fibers in contrast to few TH- and VIP-positive fibers. The short ciliary nerves also contained nNOS-positive fibers, but TH-positive fibers were more numerous than nNOS-positive ones. Parasympathetic innervation to the sweat gland is well known, but the original nerve course seemed not to be demonstrated yet. The present study may be the first report on a skin nerve containing abundant nNOS-positive fibers. The unique parasympathetic contents in the nasocilliary nerve seemed to supply the forehead sweat glands as well as glands in the eyelid and nasal epithelium.
Recently our group demonstrated a significantly greater proportion of tyrosine hydroxylase (TH)–positive, sympathetic nerve fibers in the frontal and auriculotemporal nerves than the other trigeminal nerve branches in elderly donated cadavers [1]. Therefore, parts of the human face around mouth and nose are unlikely to be innervated by sympathetic nerve fibers passing through nerve trunks but by artery-bounded nerve fibers. However, we have no information about composite fibers of cutaneous branches of the nasociliary nerve. During the proximal course in the orbit, the nasociliary nerve is richly communicated with the ciliary ganglion as well as the short ciliary nerves [2345]. Moreover, the human ciliary ganglion contains both TH-positive neurons and neuronal nitric oxide synthase (nNOS)-positive neurons [6]. Therefore, being different from the frontal nerve, the nasociliary nerve is likely to contain both sympathetic and parasympathetic nerve fibers.
A change in the human forehead skin circulation is known in response to arousal emotion to increase sympathetic nerve activation [78]. The forehead is innervated by the frontal and nasociliary nerves: both are branches of the first division of the trigeminal nerve. Near the forehead, smooth muscles, and glands in the eyelid also requires parasympathetic nerve supply, but details of the nerve course were not yet described [9]. Consequently, the aim of this study was to immunohistochemically identify sympathetic and parasympathetic nerve fibers in skin nerves of the first division of the trigeminal nerve.
The study was performed in accordance with the provisions of the Declaration of Helsinki 1995 (as revised in Edinburgh 2000) and it was approved by the Human Research Ethical Committee by the dental school. We dissected 20 cadavers donated for the annual student dissection courses at Tokyo Dental College (15 males, 5 females; 71–98 years old; mean age, 85 years old at death). The cause of death in each case had been ischemic heart failure. The donated cadavers had been fixed by intravenous injection of non-neutralized 10% (v/v) formalin solution and preserved in 50% ethanol solution for more than 3 months. From each of the cadavers, we obtained an entire right orbital content including the eyelids and a part of the forehead skin and, after routine procedures for paraffin-embedded histology, we first prepared 5-µm-thick frontal section including posterior half of the eyeball and stained with hematoxylin and eosin (Fig. 1). After identification of the nasociliary nerve as well as the frontal nerve in the posterior site, we further prepared multiple sets of sections for immunohistochemistry. Sections were provides at 4–5 sites at 1–2 mm interval toward the eye lids and forehead. For identification of the sectional planes, one of the set always included subcutaneous tissues around the trochlea of the superior obliquus muscle. In addition, at the superolateral wall of the ethmoidal sinus, we prepared a tip of the anterior ethmoidal nerve and artery from each of the cadavers.
According to Hinata et al. [10], the primary antibodies used for nerve immunohistochemistry were (1) rabbit polyclonal anti-human nNOS (1:200, Cell Signaling Technology, Beverly, MA, USA), (2) mouse monoclonal anti-human vasoactive intestinal polypeptide or vasoactive intestinal polypeptide (VIP; 1:100, sc25347, Santa Cruz Biotechnology, Santa Cruz, CA, USA), and (3) rabbit polyclonal anti-human TH (1:500, ab152, Millipore-Chemicon, Temecula, CA, USA). We tried to perform these three types of immunohistochemistry using adjacent sections, but sometimes we used near sections (not adjacent) due to a failure of the sectioning and/or immunostaining. Antigen retrieval was performed using microwave treatment (500 W, 15 minutes, pH 6). The secondary antibody (incubation for 30 minutes; 1:1,000, Histofine Simple Stain Max-PO, Nichirei, Tokyo, Japan) was labeled with horseradish peroxidase (HRP), and antigen-antibody reactions were detected by the HRP-catalyzed reaction with diaminobenzidine. Counterstaining with hematoxylin was performed on the same samples. The negative control without a first antibody was set up for each of the specimens. The short ciliary nerves in the sections were used for the positive control. Observations and taking photographs were usually performed with Nikon Eclipse 80 (Nikon, Tokyo, Japan), but photos at the ultralow magnification (less than ×1 at the objective lens) were taken using a high grade flat scanner with translucent illumination (Epson scanner GTX970; Epson, Tokyo, Japan).
Identification of the nasociliary nerve was not difficult because, at the peripheral course, it ran near the superior obliquus muscle with a concomitant artery. After finding the nerve in a posterior section including the posterior pole of the eye ball (Fig. 1), we traced it toward the eyelid and forehead skin using semiserial sections. We included the trochlea of the superior obliquus muscle into one of the sections to make sure of the sectional planes. Immunohistochemistry of nNOS was successfully conducted in 12 of 20 cadavers, but the reactivity was not seen in short ciliary nerves (positive control) in the other 8 cadavers. The results shown below will be based on the 12 cadavers with successful results.
In the available 12 specimens, throughout the peripheral course from a level of the posterior pole of the eyeball, via a site near the trochlea and toward the skin, the nasociliary nerve consistently contained abundant nNOS-positve fibers. However, VIP-positive fibers were always few in number (Figs. 2, 3). TH-positive fibers considerably varied in number between specimens. We did not find a difference in the nNOS immunoreactivity between sites before and after an origin of the anterior ethmoidal nerve from the nasociliary nerve. Actually, we did not find nNOS reactivity in a fragment including the anterior ethmoidal nerve and artery (data not shown). The frontal nerve always contained abundant TH-positive fiber, but no or few VIP- and nNOS-positive fibers were included. The short ciliary nerves contained all three types of fibers. Although we found individual variations in TH-positive fiber contents, there appeared not to be the correlation with age or gender. Because of the longitudinal or oblique sections of nerve fibers, the morphometrical analysis was difficult.
Many research groups had paid attention to a critical role of nitric oxide synthase to control blood supply in the skin [11121314]. However, an immunohistochemical demonstration of nNOS along the cutaneous nerve course seemed to be limited to Ibba-Manneschi et al. [15]. Using frozen sections of skin specimens from systemic sclerosis patients and healthy volunteers, they displayed a lower magnification views of subcutaneous nerves running toward the dermis. However, in the figures, we were not able to identify the positive nerve fibers or the other associated cells. Schulze et al. [16] demonstrated a co-existence of NOS and VIP in cutaneous nerve fibers in human face and forearm, but their results were not based on immunohistochemistry but enzyme histochemistry of NOS. Therefore, the present study might be the first demonstration of nNOS-positive nerve fibers in and along a skin nerve course.
A significant difference in composite nerve fibers between the frontal and nasociliary nerves seemed to be explained by a fact that the latter nerve is most likely to supply nNOS-positive fibers to smooth muscles and glands in the eyelid and upper nasal epithelium. However, the nNOS-positive fibers were also likely to play a role in emotional sweating in the forehead [78]. In contrast to sympathetic nerve fibers to skin [1718], it was difficult to find a report of nNOS expression in skin nerves. We had also conducted many immunohistochemical studies of the human perineum, but we did not find such fibers in skin nerves from the pudendal nerve [1019]. Likewise, in contrast to TH-positive fibers to skeletal muscles [20], Hosaka et al. [21] did not find nNOS-positive fibers in muscle nerves in the human body.
One reason of no or few reports on the parasympathetic peripheral nerve course was found in a difficulty in nNOS immunostaining using specimens from formalin-treated donated cadavers after long preservation [22]. Another reason seemed to exist in the so-called cholinergic sympathetic innervation [23]. Cholinergic parasympathetic fibers can express kinds of sympathetic nerve markers such as neuropeptide Y and/or dopamine-beta-hydroxylase [24]. However, according to our long trials, the cadaveric specimens after long preservation are not available for immunostainings of choline acetyltransferease, neuropeptide Y, and dopaminebeta-hydroxylase. This is a critical study limitation of this study. A study using biopsy specimens from volunteers could bring further information of parasympathetic nerve course to the skin. A new technique such as signal amplification systems [25] may be helpful.
Figures and Tables
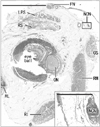 | Fig. 1Topographical anatomy of the nasociliary and frontal nerves (a 78-year-old woman). Frontal section of the right eye. Hematoxylin and eosin staining. In the present study, the nasociliary nerve (NCN) was identified in a large frontal section including the posterior pole of the eye ball (scale bar=10 mm) and we traced the nerve anteriorly toward the skin. An insert at the lower angle is a higher magnification view of a square including the nasociliary nerve (scale bar=1 mm). FN, frontal nerve; LPS, levator palpebrae superioris muscle; ON, optic nerve; OS, obliquus superior muscle; RI, rectus inferior muscle; RL, rectus lateralis muscle; RM, rectus medialis muscle; RS, rectus superior muscle. |
 | Fig. 2Immunohistochemistr y of nerves (a 78-year-old woman). The specimen same as in Fig. 1. Frontal sections almost 2 mm anterior to Fig. 1. (A–D) The nasocilliary nerve (A–C, near sections). (E–G) A ciliary nerve (near sections). (H–J) The frontal nerve (near sections). (A, D, E, H) Immunohistochemistry of neuronal nitric oxide synthase (NOS). (B, F, I) Immunohistochemistry of vasoactive intestinal polypeptide (VIP). (C, G, J) Immunohistochemistry of tyrosine hydroxylase (TH). The nasociliary nerve contains abundant NOS-positive nerve fibers, while TH-positive fibers are dominant in the frontal nerve. A branch of the nasociliary nerve contains NOS-positive ganglion cells (D). All panels are prepared at the same magnification. Scale bar=0.1 mm. |
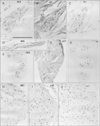 | Fig. 3Immunohistochemistry of nerves (an 89-year-old man). Frontal sections. (A–D) The nasocilliary nerve (A–C, near sections). (E–G) A ciliary nerve (near sections). (H–J) The frontal nerve (near sections). (A, D, E, H) Immunohistochemistry of neuronal nitric oxide synthase (NOS). (B, F, I) Immunohistochemistry of vasoactive intestinal polypeptide (VIP). (C, G, J) Immunohistochemistry of tyrosine hydroxylase (TH). The nasocilliary nerve contain abundant NOS-positive nerve fibers, while TH-positive fibers are dominant in the frontal nerve. (D) A branch of the nasocilliary nerve. All panels are prepared at the same magnification. Scale bar=0.1 mm. |
Acknowledgements
We are grateful to the individuals who donated their bodies after death to Tokyo Dental College for research and education on human anatomy without any economic benefit. We also thank their families for agreeing to the donation as well as their patience in waiting for the return of their remains after study.
References
1. Matsubayashi T, Cho KH, Jang HS, Murakami G, Yamamoto M, Abe SI. Significant differences in sympathetic nerve fiber density among the facial skin nerves: a histologic study using human cadaveric specimens. Anat Rec (Hoboken). 2016; 04. 12. [Epub]. DOI: 10.1002/ar.23347.
2. Baljet B, van der Werf F, Otto AJ. Autonomic pathways in the orbit of the human fetus and the rhesus monkey. Doc Ophthalmol. 1989; 72:247–264.
3. Oikawa S, Kawagishi K, Yokouchi K, Fukushima N, Moriizumi T. Immunohistochemical determination of the sympathetic pathway in the orbit via the cranial nerves in humans. J Neurosurg. 2004; 101:1037–1044.
4. Thakker MM, Huang J, Possin DE, Ahmadi AJ, Mudumbai R, Orcutt JC, Tarbet KJ, Sires BS. Human orbital sympathetic nerve pathways. Ophthal Plast Reconstr Surg. 2008; 24:360–366.
5. Rusu MC, Pop F. The anatomy of the sympathetic pathway through the pterygopalatine fossa in humans. Ann Anat. 2010; 192:17–22.
6. Kiyokawa H, Katori Y, Cho KH, Murakami G, Kawase T, Cho BH. Reconsideration of the autonomic cranial ganglia: an immunohistochemical study of mid-term human fetuses. Anat Rec (Hoboken). 2012; 295:141–149.
7. Nordin M. Sympathetic discharges in the human supraorbital nerve and their relation to sudo- and vasomotor responses. J Physiol. 1990; 423:241–255.
8. Cramer MN, Bain AR, Jay O. Local sweating on the forehead, but not forearm, is influenced by aerobic fitness independently of heat balance requirements during exercise. Exp Physiol. 2012; 97:572–582.
9. Yuzuriha S, Matsuo K, Ishigaki Y, Kikuchi N, Kawagishi K, Moriizumi T. Efferent and afferent innervations of Mueller's muscle related to involuntary contraction of the levator muscle: important for avoiding injury during eyelid surgery. Br J Plast Surg. 2005; 58:42–52.
10. Hinata N, Hieda K, Sasaki H, Murakami G, Abe S, Matsubara A, Miyake H, Fujisawa M. Topohistology of sympathetic and parasympathetic nerve fibers in branches of the pelvic plexus: an immunohistochemical study using donated elderly cadavers. Anat Cell Biol. 2014; 47:55–65.
11. Kellogg DL Jr, Zhao JL, Wu Y. Neuronal nitric oxide synthase control mechanisms in the cutaneous vasculature of humans in vivo. J Physiol. 2008; 586:847–857.
12. Ikeyama K, Denda S, Tsutsumi M, Denda M. Neuronal nitric oxide synthase in epidermis is involved in cutaneous circulatory response to mechanical stimulation. J Invest Dermatol. 2010; 130:1158–1166.
13. Del Pozzi AT, Carter SJ, Collins AB, Hodges GJ. The regional differences in the contribution of nitric oxide synthase to skin blood flow at forearm and lower leg sites in response to local skin warming. Microvasc Res. 2013; 90:106–111.
14. Wong BJ. Sensory nerves and nitric oxide contribute to reflex cutaneous vasodilation in humans. Am J Physiol Regul Integr Comp Physiol. 2013; 304:R651–R656.
15. Ibba-Manneschi L, Niissalo S, Milia AF, Allanore Y, Del Rosso A, Pacini A, Manetti M, Toscano A, Cipriani P, Liakouli V, Giacomelli R, Kahan A, Konttinen YT, Matucci-Cerinic M. Variations of neuronal nitric oxide synthase in systemic sclerosis skin. Arthritis Rheum. 2006; 54:202–213.
16. Schulze E, Witt M, Fink T, Hofer A, Funk RH. Immunohistochemical detection of human skin nerve fibers. Acta Histochem. 1997; 99:301–309.
17. Balogh B, Auterith A, Behrus R, Maier S, Vesely M, Piza-Katzer H. The sympathetic axons of the nerves of the hand. Handchir Mikrochir Plast Chir. 2002; 34:369–373.
18. Marx SC, Kumar P, Dhalapathy S, Marx CA, D'Souza AS. Distribution of sympathetic fiber areas of radial nerve in the forearm: an immunohistochemical study in cadavers. Surg Radiol Anat. 2010; 32:865–871.
19. Kinugasa Y, Arakawa T, Murakami G, Fujimiya M, Sugihara K. Nerve supply to the internal anal sphincter differs from that to the distal rectum: an immunohistochemical study of cadavers. Int J Colorectal Dis. 2014; 29:429–436.
20. Nakao T, Cho KH, Yamamoto M, Yamane S, Murakami G, Ide Y, Abe S. Site-dependent difference in the density of sympathetic nerve fibers in muscle-innervating nerves: a histologic study using human cadavers. Eur J Anat. 2012; 16:33–42.
21. Hosaka F, Katori Y, Kawase T, Fujimiya M, Ohguro H. Site-dependent differences in density of sympathetic nerve fibers in muscle-innervating nerves of the human head and neck. Anat Sci Int. 2014; 89:101–111.
22. Hieda K, Cho KH, Arakawa T, Fujimiya M, Murakami G, Matsubara A. Nerves in the intersphincteric space of the human anal canal with special reference to their continuation to the enteric nerve plexus of the rectum. Clin Anat. 2013; 26:843–854.
23. Guidry G, Landis SC. Absence of cholinergic sympathetic innervation from limb muscle vasculature in rats and mice. Auton Neurosci. 2000; 82:97–108.
24. Hardebo JE, Suzuki N, Ekblad E, Owman C. Vasoactive intestinal polypeptide and acetylcholine coexist with neuropeptide Y, dopamine-beta-hydroxylase, tyrosine hydroxylase, substance P or calcitonin gene-related peptide in neuronal subpopulations in cranial parasympathetic ganglia of rat. Cell Tissue Res. 1992; 267:291–300.
25. Wang N, Gibbons CH, Freeman R. Novel immunohistochemical techniques using discrete signal amplification systems for human cutaneous peripheral nerve fiber imaging. J Histochem Cytochem. 2011; 59:382–390.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download