Abstract
The raphe of the human penis and scrotum is considered to develop secondarily after disappearance of the initial midline seam by fusion of the bilateral genital folds. However, the fetal development was still obscure. We examined histological sections of 30 fetuses (17 males and 13 females) at 10–15 weeks. In male fetuses, the scrotum was not yet clearly identified because of no descent of testis. The perineal raphe was thin and wavy at 10 weeks, and it was continuous with and took a direction same as the inferior wall of the closed penile urethra after physiological hypospadias. Depending on growth of the bulbospongiosus muscle and corpus spongiosus penis, the midline intermuscular septum obtained a connection to the subcutaneous wavy raphe and made the latter thick and straight at 12–15 weeks. Notably, the perineal raphe extended posteriorly to attach to the external anal sphincter. In female fetuses, an epithelial fusion occurred along a short distance at the posterior end of the vestibule. However, in front of the external anal sphincter, a large midline mesenchymal tissue from the urorectal septum did not contain a raphe-like structure. Moreover, since the bilateral bulbospongiosus muscles were separated widely by the vestibule, they did not provide a midline septum. Fetal development of the perineal raphe was accelerated by reinforcement from the muscular septum. In contrast, without such a muscular support, the female raphe could not maintain its growth even if the seed appeared at the posterior end of the vestibule.
The penile and scrotal raphe is a subcutaneous fibrous plate extending from the penile frenulum, along the penile shaft and scrotum and toward the anus. In mice, the urorectal septum of endodermal origin plays a role of zipper to close the bilateral genital folds [12]. Thus, in mice, the midline seam or raphe is originated from the urorectal septum. However, since physiological hypospadias is evident in human fetuses, a contribution of the urorectal septum is still unclear in the penile raphe. An epithelial-mesenchymal interaction has been hypothesized for the delayed fusion under control of androgen [34]. In contrast, in the human perineum between the scrotum and anus, the urorectal septum is most likely to provide the perineal raphe as in mice. In human mid-term fetuses, a future scrotum was difficult to identify because of no descent of testis [5]. Thus, in the present study, we will apply a term "perineal raphe" to a raphe behind the root of penis.
Early development of the anus from the cloaca has been a major subject in embryology [67891011]. However, to our knowledge, only one researcher [12] described details of fetal development of the raphe possibly due to early stages observed by the other researchers. According to him, the human raphe appears at 9 weeks of gestation and becomes thicker and continuous with the anus at 12–13 weeks. Notably, van der Putte [12] did not consider the raphe as a remnant of fusion of the bilateral genital folds but a secondary formation after disappearance of the latter. Therefore, the first aim of this study was to describe fetal development of the male perineal raphe behind the root of penis.
In adults and children, the penile and scrotal raphe vary in shape and thicknmess between individuals and the anomalies include pearly, pigmented, prominent, or wide raphe [13]. The anomalies sometimes include a "skin groove" extending from the hypospadic urethra to the anus [1415]. Likewise, the perineal groove is known as a rare female anomaly consisting of a wet skin grove from the vestibule to the anus. This anomaly has been considered to result from (1) a failure of the genital folds to fuse, (2) a relic of the open cloaca, and (3) a failure of the urorectal septum to develop downward [1617]. In this context, a midline fusion of the bilateral genital folds seems to be hypothesized in the female perineum. However, the female genital folds are not able to fuse together and, instead, they are widely separated by the vestibule in female fetuses [18]. Surprisingly, in both genders, a thick perineal raphe or its variation is often evident in combination with various types of anorectal malformations [19]. Therefore, the second aim of this study was to examine whether the raphelike structure was present or absent behind the fetal vestibule. In the mid-term human fetus, the vagina does not descend to reach the vestibule [20].
The study was performed in accordance with the provisions of the Declaration of Helsinki 1995 (as revised in Edinburgh 2000). We observed serial paraffin sections (5–10µm in thickness) of 30 fetuses (17 males and 13 females; crown-rump length 48–115 mm or approximately 10–15 weeks): they were composed of 9 males and 4 females at 10 weeks, 4 males and 5 females at 12–13 weeks, and 4 males and 4 females at 14–15 weeks. The sectional planes were frontal (20 specimens) or sagittal (10 specimens). Since these sagittal sections were slightly tilted to the frontal planes, midline structures such as the symphysis pubis and perineal raphe were able to be identified as linear structures. The sections had been stained with hematoxylin and eosin, Masson trichrome or azan as a part of the Medical Museum Collection, Georg- August-Universität Göttingen (4 specimens, all sagittal) and Institute of Embryology, Universidad Complutense Madrid (the other 26 specimens).
For observations of the Blechschmidt collection in the Medical Museum of Georg-August-Universität Göttingen [21], we did not need approval by the ethics committee of the institute. Our use of sections in the collection kept at the Institute of Embryology, Universidad Complutense Madrid [22] was approved by the university ethics committee (No. B08/374). Observations and taking photographs were performed with Nikon Eclipse 80 (Nikon, Tokyo, Japan) in the museum or institute.
At 10 weeks, the perineal raphe was present in the superficial or posterior side of the proximal urethra. Notably, the initial raphe was continuous with and took a direction same as the inferior or posterior wall of the urethra after closure of physiological hypospadias (Fig. 1). In the collection in Madrid, we did not find a cross section of the penile urethra. Thus, we were not able to demonstrate clearly an absence of the penile raphe at the early stage. However, the penile urethra appeared to be as short as the initial perineal raphe (Fig. 1A vs. Fig. 1D). Depending on the tilting of sectional plane, the future scrotum was sometimes identified as a thick subcutaneous tissue in the inferior side of the pubic ramus (Fig. 1E). Since the thickened tissue was often difficult to identify because of no descent of testis (Fig. 2), instead of the term "scrotum," we will use "the root of penis" in the observations below. Near the anus, a midline septum was sandwiched by the bilateral corpus spongiosum penis as well as by the bilateral bulbospongiosus muscles (Fig. 2A–D).
At 12–13 weeks, the raphe became distinct but wavy in the subcutaneous tissue at and behind the root of penis (Fig. 2E–H). The bulbospongiosus muscle increased in mass and extended laterally toward the ischiocavernosus muscle and posteriorly toward the external anal sphincter (EAS) (Fig. 2F, G). However, in those specimens at 10–13 weeks, the EAS was still thin and small and identified as scattering muscle fibers in the subcutaneous tissue (Fig. 2D, G). At 14–15 weeks, the perineal raphe as well as its deep continuation (i.e., the intermuscular septum) was thick and almost straight, depending on the extent of growth of the bulbospongiosus muscles (Fig. 3). The posterior parts of the muscle were attached to and communicated with the EAS (Figs. 3C, 4). The perineal raphe ended at the anterior margin of the EAS (Figs. 3D, 4E). The urethral groove of the penis was completely closed until 12 weeks (i.e., closure of physiological hypospadias). In addition, throughout the ages examined, an intermuscular space was present between the bulbospongiosus and ischiocavernosus muscles (Figs. 2A, E, 3B, 4E).
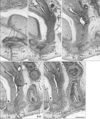
In all female specimens examined, the vaginal descent along the urogenital sinus or urethra was not completed: the inferior end of the vagina was still above the level of the inferior margin of the pubic symphysis. At 10–13 weeks, the large phallus or initial clitoris protruded anteriorly, making it difficult to distinguish from a penis with physiological hypospadias (Fig. 5). The inferior end of the vagina or the sinovaginal bulb was identified as a solid tissue mass on the posterosuperior side of the vestibule (Figs. 5A, 6, 7). In 2 specimens, we found a short epithelial fusion or seam at the posterior end of the vestibule (Fig. 5C), but this midline fusion did not connect with the bulbospongiosus muscle. At 14–15 weeks, the muscle elongated superiorly and posteriorly along the vestibular bulb (Fig. 6).
In all specimens, the bilateral bulbospongiosus muscles were separated widely by the vestibule (Figs. 5D, 6B, C), except for the anterosuperior end near the crus clitoris. The muscle did not attach to the EAS, but there was a loose tissue mass interposed in front of the EAS (Fig. 7). As described in males, an intermuscular space was consistently seen between the bulbospongiosus and ischiocavernosus muscles (Figs. 5A, 6C). Overall, there was no evidence of a raphe-like structure in the fetal female perineum. The gender difference in topographical anatomy of the bulbospongiosus muscle and raphe is schematically represented in Fig. 8. Finally, we did not find a candidate of the perineal body in the present fetuses.
In the present study, we incidentally found a short epithelial fusion or seam at the posterior end of the vestibule. However, in front of the EAS, a large midline mesenchymal tissue did not contain a raphe-like structure. In the male perineum, the raphe grew as a superficial extension of the midline or intermuscular septum between the bilateral bulbospongiosus muscles. The raphe always reached the EAS. Conversely, according to Arakawa et al. [23], the subcutaneous part of the EAS tends to develop near and along the perineal raphe in male fetuses [23]. Therefore, an abnormal raphe was likely combined with abnormal perineal muscles including the EAS. In the female perineum, the bilateral muscles were separated widely by the vestibule. Without reinforcement by the midline muscular septum, the female raphe could not maintain its growth even if the seed appeared at the posterior end of the vestibule. This situation was somewhat similar to a difficulty in fetal development of the female circular rhabdosphincter [1820]. Due to a long distance to the bulbospongiosus muscles, a major driving force for growth of the penile raphe might be an increased thickness of the septum between the bilateral corpus cavernosum penis.
In relation to fetal development of perineal midline structures in females, we introduced an anomalous perineal groove (see the "Introduction"). In female fetuses, a large mesenchymal tissue from the urorectal septum existed in front of the EAS and it appeared not to be involved in the growing vestibule. Therefore, rather than a failure of the genital folds to fuse, a failure of the urorectal septum to develop downward seemed to cause the abnormality in the female perineum. Because it is often lined by rectal columnar epithelium, not by the squamous epithelium [16], the perineal groove seemed not to be a relic of the open cloacal duct. The developing anal sinus is likely to contribute to provide the columnar epithelium of the groove since a long sinus sometimes extends anteriorly to reach the smooth muscle wall [24]. In addition, a candidate of the perineal body was not found in the present fetuses since it is originated from the longitudinal smooth muscle layer of the rectum at 20 weeks or later [2325].
A great limitation of the present study was found in lack of suitable transverse sections of the penis at 10–12 weeks. Using such sections, a further study was necessary to ensure no penile raphe immediately after the closure of physiological hypospadias. However, because of sections with a routine staining, it seemed to be difficult to deny a possibility that, after epithelial-mesenchymal transition, the epithelial seam for closure differentiates into the initial penile raphe.
Figures and Tables
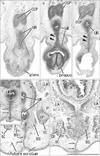 | Fig. 1Early perineal raphe and the urethral wall in a 10-week male fetus without a definite penile raphe. Frontal sections. Hematoxylin and eosin staining. Panel (A), including a longitudinal course of the penile urethra (UR), displays the most anterior side of photos. Intervals between panels are 0.1 mm (A–B, B–C), 0.2 mm (C–D, D–E), and 0.5 mm (E–F), respectively. (B, C) The inferior or posterior margin of the urethral wall after closure (arrowheads). (D, E) The perineal raphe connecting between the UR and surface skin (arrows). The perineal raphe is continuous with and takes a direction same as the penile UR. (F) Near the rectum (R), the raphe (arrows) is continuous with a developing midline septum of the bilateral bulbospongiosus muscles (BS). CCP, corpus cavernosum penis; CP, crus penis; IC, ischiocavernosus muscle; LA, levator ani muscle; P, pubis; RS, rhabdosphincter. All panels were prepared at the same magnification. Scale bar=1 mm. |
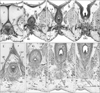 | Fig. 2Early perineal raphe seen in two male fetuses. Frontal sections. Hematoxylin and eosin staining. (A–D) A 10- week fetus. (E–H) A 12-week fetus. (A, E) The most anterior side of each series of photos. Intervals between panels are 0.3 mm (A–B, B–C), 0.4 mm (C–D), and 0.6 mm (E–F, F–G, G–H), respectively. Arrows indicate the perineal raphe connecting between the inferior aspect of the urethra and the surface skin. The raphe disappears in the immediately anterior side of the external anal sphincter (EAS). The bulbospongiosus muscle (BS) is small and contains bleeding (A–C), while the bilateral muscles attaches the midline septum (E–G). (G) The putative Cowper's gland (gland) starts development. (A, E) An intermuscular space (star) between the BS and ischiocavernosus muscle (IC). BL, bladder; CP, crus penis; CSP, corpus spongiosum penis; DN, dorsal nerve of the penis or clitoris; LA, levator ani muscle; OI, obturator internus muscle; P, pubis; R, rectum; RS, rhabdosphincter; UR, urethra. All panels were prepared at the same magnification. Scale bar=1 mm. |
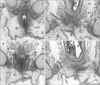 | Fig. 3Midline septum between the bilateral bulbospongiosus muscles (BS) in a 14-week male fetus. Frontal sections. Hematoxylin and eosin staining. Panel A (panel D) displays the most anterior (posterior) side of the figure. Intervals between panels are 0.5 mm (A–B), 0.4 mm (B–C), and 0.1 mm (C–D), respectively. In the inferoposterior side of the urethra (UR), a midline septum between the bilateral BS extend to the subcutaneous raphe (arrows). (C, D) The raphe is interrupted by and ends at the anterior margin of the external anal sphincter (EAS). Stars indicate an intermuscular space between the BS and ischiocavernosus muscle (IC). CP, crus penis; CSP, corpus spongiosum penis; LA, levator ani muscle; OI, obturator internus muscle; P, pubis; PN, pudendal nerve; R, rectum; SC, scrotum. All panels were prepared at the same magnification. Scale bar=1 mm. |
 | Fig. 4Perineal raphe seen in the tilted sagittal sections of a 15-week male fetus. Tilted sagittal sections. Hematoxylin and eosin staining. Panel A (panel E) displays the most lateral anterior (medial) side of the figure. Intervals between panels are 0.8 mm (A–B), 0.4 mm (B–C), and 0.2 mm (C–D, D–E), respectively. (A) Because of tilted sections, the root of the penis is cut almost frontally and the raphe (arrows) is seen without associated muscles. (B) The raphe appears to connect with a septum in the corpus spongiosum penis (CSP). (E) The raphe disappears at the anterior margin of the external anal sphincter (EAS). Stars indicate an intermuscular space between the bulbospongiosus muscle (BS) and ischiocavernosus muscle (IC). BL, bladder; CCP, corpus cavernosum penis; CP, crus penis; DN, dorsal nerve of the penis or clitoris; LA, levator ani muscle; P, pubis; PC, peritoneal cavity; PR, prostate; PX, pelvic autonomic nerve plexus; R, rectum; RS, rhabdosphincter; SV, seminal vesicle; UR, urethra; VAS, vas deferens. All panels were prepared at the same magnification. Scale bar=1 mm. |
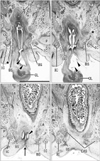 | Fig. 5Clitoris protruding anteriorly and an area in the posterior side of the vestibule in a 12-week female fetus. Frontal sections. Hematoxylin and eosin staining. Panel A (panel D) displays the most anterior (posterior) side of the figure. Intervals between panels are 0.2 mm (A–B), 0.7 mm (B–C), and 0.5 mm (C–D), respectively. (A) The vestibule (arrowheads) as well as the vagina connecting to the distalmost urethra (UR). (B) The vestibule opens. (C) In the posterior margin of the vestibule, an epithelial fusion provides a short raphe-like structure (arrow). (D) However, no raphe is seen in a loose tissue occupyng between the vestibule and anus. (A) An intermuscular space (star) between the bulbospongiosus muscle (BS) and ischiocavernosus muscle (IC). CC, crus clitoris; CL, clitoris; EAS, external anal sphincter; LA, levator ani muscle; OI, obturator internus muscle; P, pubis; PX, pelvic autonomic nerve plexus; R, rectum; VAG, vagina. All panels were prepared at the same magnification. Scale bar=1 mm. |
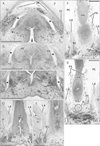 | Fig. 6Bilateral bulbospongiosus muscles (BS) are separated by the vestibule and urethra (UR) in a 15- week female fetus. Frontal sections. Hematoxylin and eosin staining (the color is changed in panel B). Panel A (panel G) displays the most anterior (posterior) side of the figure. Intervals between panels are 0.3 mm (A–B, B–C), 0.4 mm (C–D), and 0.2 mm (D–E, E–F, and F–G), respectively. (A) The vestibule opens inferiorly (arrowhead). (A–D) Bilateral BS are separated by the midline vestibule and UR. (E, F) The vagina (VAG) attaches to the UR at a site indicated by an open star. (G) The longitudinal smooth muscles of the rectum (R with circle). A raphe-like structure is absent in the posterior side of the vestibule. Black stars indicate an intermuscular space between the BS and ischiocavernosus muscle (IC). CC, crus clitoris; DN, dorsal nerve of the penis or clitoris; EAS, external anal sphincter; LA, levator ani muscle; OI, obturator internus muscle; P, pubis; PC, peritoneal cavity; PX, pelvic autonomic nerve plexus; R, rectum; VB, vestibular bulb. Scale bars=1 mm (A–G) |
 | Fig. 7Developing vestibule in tilted sagittal sections of a 12-week female fetus. Tilted sagittal sections. Hematoxylin and eosin staining. (B) Because of the symphysis pubis (arrowheads) is identified as a linear structure, the sagittal planes are tilted to the frontal planes. Panel (D) corresponds to the almost midplane, while the other panels are lateral. Intervals between panels are 0.2 mm (A–B), 0.4 mm (B–C), 0.2 mm (C–D), 0.05 mm (D–E), and 0.2 mm (E–F), respectively. (C, D) The vagina descends to a level of the inferior margin of the pubis. (C–E) The vestibule opens inferiorly (arrowheads). The bulbospongiosus muscle (BS) attaches to the vestibular bulb (VB). (A, B) A tissue damage (asterisks) during histological procedure. A raphe-like structure is absent in an area (triangle) between the vestibule and the external anal sphincter (EAS). BL, bladder; CC, crus clitoris; CL, clitoris; DN, dorsal nerve of the penis or clitoris; IC, ischiocavernosus muscle; P, pubis; PX, pelvic autonomic nerve plexus; R, rectum; RS, rhabdosphincter; UR, urethra; VAG, vagina. All panels were prepared at the same magnification. Scale bar=1 mm. |
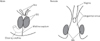 | Fig. 8A diagram showing a gender difference in topographical anatomy of the bilateral bulbospongiosus muscle. In male fetuses, the bilateral bulbospongiosus muscles (BS) makes a midline intermuscular septum and reinforces the perineal raphe. In female fetuses, the bilateral BS are widely separated by the vestibule opening inferiorly. |
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81460471).
References
1. Seifert AW, Harfe BD, Cohn MJ. Cell lineage analysis demonstrates an endodermal origin of the distal urethra and perineum. Dev Biol. 2008; 318:143–152.
2. Georgas KM, Armstrong J, Keast JR, Larkins CE, McHugh KM, Southard-Smith EM, Cohn MJ, Batourina E, Dan H, Schneider K, Buehler DP, Wiese CB, Brennan J, Davies JA, Harding SD, Baldock RA, Little MH, Vezina CM, Mendelsohn C. An illustrated anatomical ontology of the developing mouse lower urogenital tract. Development. 2015; 142:1893–1908.
3. Baskin LS, Erol A, Jegatheesan P, Li Y, Liu W, Cunha GR. Urethral seam formation and hypospadias. Cell Tissue Res. 2001; 305:379–387.
4. Kim KS, Liu W, Cunha GR, Russell DW, Huang H, Shapiro E, Baskin LS. Expression of the androgen receptor and 5 alphareductase type 2 in the developing human fetal penis and urethra. Cell Tissue Res. 2002; 307:145–153.
5. Niikura H, Okamoto S, Nagase S, Takano T, Murakami G, Tatsumi H, Yaegashi N. Fetal development of the human gubernaculum with special reference to the fasciae and muscles around it. Clin Anat. 2008; 21:547–557.
6. de Vries PA, Friedland GW. The staged sequential development of the anus and rectum in human embryos and fetuses. J Pediatr Surg. 1974; 9:755–769.
7. Kluth D, Hillen M, Lambrecht W. The principles of normal and abnormal hindgut development. J Pediatr Surg. 1995; 30:1143–1147.
8. Nievelstein RA, van der Werff JF, Verbeek FJ, Valk J, Vermeij-Keers C. Normal and abnormal embryonic development of the anorectum in human embryos. Teratology. 1998; 57:70–78.
9. Yamaguchi K, Kiyokawa J, Akita K. Developmental processes and ectodermal contribution to the anal canal in mice. Ann Anat. 2008; 190:119–128.
10. van der Putte SC. The development of the human anorectum. Anat Rec (Hoboken). 2009; 292:951–954.
11. Zhang T, Zhang HL, Wang DJ, Tang XB, Jia HM, Bai YZ, Yuan ZW, Wang WL. Normal development of hindgut and anorectum in human embryo. Int J Colorectal Dis. 2011; 26:109–116.
12. van der Putte SC. The devlopment of the perineum in the human. A comprehensive histological study with a special reference to the role of the stromal components. Adv Anat Embryol Cell Biol. 2005; 177:1–131.
13. Baky Fahmy MA. The spectrum of genital median raphe anomalies among infants undergoing ritual circumcision. J Pediatr Urol. 2013; 9(6 Pt A):872–877.
14. Abdel Aleem A, el Sheikh S, Mokhtar A, Ghafouri H, Saleem M. The perineal groove and canal in males and females: a third look. Z Kinderchir. 1985; 40:303–307.
15. Chatterjee SK, Chatterjee US, Chatterjee U. Perineal groove with penoscrotal hypospadias. Pediatr Surg Int. 2003; 19:554–556.
16. Mullassery D, Turnock R, Kokai G. Perineal groove. J Pediatr Surg. 2006; 41:e41–e43.
17. Sekaran P, Shawis R. Perineal groove: a rare congenital abnormality of failure of fusion of the perineal raphe and discussion of its embryological origin. Clin Anat. 2009; 22:823–825.
18. Masumoto H, Takenaka A, Rodríguez-Vázquez JF, Murakami G, Matsubara A. Reappraisal of intergender differences in the urethral striated sphincter explains why a completely circular arrangement is difficult in females: a histological study using human fetuses. Anat Cell Biol. 2012; 45:79–85.
19. Murphy F, Puri P, Hutson JM, Holschneider AM. Incidence and frequency of different types, and classification of anorectal malformations. In : Holschneider AM, Hutson JM, editors. Anorectal Malformations in Children. Berlin: Springer;2006. p. 163–184. .
20. Masumoto H, Rodríguez-Vázquez JF, Verdugo-López S, Murakami G, Matsubara A. Fetal topographical anatomy of the female urethra and descending vagina: a histological study of the early human fetal urethra. Ann Anat. 2011; 193:500–508.
21. Radlanski RJ, Renz H, Klarkowski MC. Prenatal development of the human mandible. 3D reconstructions, morphometry and bone remodelling pattern, sizes 12-117 mm CRL. Anat Embryol (Berl). 2003; 207:221–232.
22. Rodríguez-Vázquez JF, Mérida-Velasco JR, Verdugo-López S, Sánchez-Montesinos I, Mérida-Velasco JA. Morphogenesis of the second pharyngeal arch cartilage (Reichert's cartilage) in human embryos. J Anat. 2006; 208:179–189.
23. Arakawa T, Hayashi S, Kinugasa Y, Murakami G, Fujimiya M. Development of the external anal sphincter with special reference to intergender difference: observations of mid-term fetuses (15-30 weeks of gestation). Okajimas Folia Anat Jpn. 2010; 87:49–58.
24. Arakawa T, Hwang SE, Kim JH, Wilting J, Rodríguez-Vázquez JF, Murakami G, Hwang HP, Cho BH. Fetal growth of the anal sinus and sphincters, especially in relation to anal anomalies. Int J Colorectal Dis. 2016; 31:493–502.
25. Hirata E, Fujiwara H, Hayashi S, Ohtsuka A, Abe S, Murakami G, Kudo Y. Intergender differences in histological architecture of the fascia pelvis parietalis: a cadaveric study. Clin Anat. 2011; 24:469–477.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download