Abstract
Treatment with cisplatin for cancer therapy has a major side effect such as nephrotoxicity; however, the role of poly (ADP-ribose) polymerase 1 (PARP1) in necrosis in response to cisplatin nephrotoxicity remains to be defined. Here we report that cisplatin induces primary necrosis through PARP1 activation in kidney proximal tubular cells derived from human, pig and mouse. Treatment with high dose of cisplatin for 4 and 8 hours induced primary necrosis, as represented by the percentage of propidium iodide-positive cells and lactate dehydrogenase release. The primary necrosis was correlated with PARP1 activation during cisplatin injury. Treatment with PJ34, a potent PARP1 inhibitor, at 2 hours after injury attenuated primary necrosis after 8 hours of cisplatin injury as well as PARP1 activation. PARP1 inhibition also reduced the release of lactate dehydrogenase and high mobility group box protein 1 from kidney proximal tubular cells at 8 hours after cisplatin injury. Oxidative stress was increased by treatment with cisplatin for 8 hours as shown by 8-hydroxy-2'-deoxyguanosine and lipid hydroperoxide assays, but PARP1 inhibition at 2 hours after injury reduced the oxidative damage. These data demonstrate that cisplatin-induced PARP1 activation contributes to primary necrosis through oxidative stress in kidney proximal tubular cells, resulting in the induction of cisplatin nephrotoxicity and inflammation.
Cisplatin and related platinum-based therapeutics are widely used for treatment of malignant tumors in testis, ovary, breast and many other organs [1, 2]. Although cisplatin is efficacious against cancer, its use is limited by severe side effects in normal tissue; among them, the major side effect is nephrotoxicity [3]. After cisplatin administration, approximately 34% patients develop renal dysfunction manifested by lower glomerular filtration rate and higher serum creatinine, resulting in acute kidney injury [1, 4]. The side effects of cisplatin, especially nephrotoxicity, remain a significant problem that restricts the use and efficacy of cisplatin in cancer therapy.
Renal tubular cell death is a common feature of cisplatin nephrotoxicity. During the past half century, it has been well recognized that proximal and distal tubular segments are the major sites of cell death during cisplatin nephrotoxicity [5, 6]. However, a recent study has shown that proximal tubules have significantly more susceptibility to cell death induced by cisplatin nephrotoxicity, compared to that in distal tubules [7]. In in vivo cisplatin nephrotoxicity models, both necrotic and apoptotic cell death are identified [8]. In cultured kidney tubule epithelial cells in vitro, an earlier finding suggested that both apoptotic and necrotic cell death are dependent on cisplatin concentration [9]. Necrotic cell death was detected when cells were treated with high concentrations of cisplatin, termed primary necrosis; while treatment with lower concentrations of cisplatin induced apoptotic cell death. However, necrosis can also be a postmortem result of apoptotic cell death, termed secondary necrosis [10]. Recent studies have focused on the mechanisms of apoptosis induced by cisplatin in kidney tubular cells [11, 12, 13], but the role of cisplatin in necrosis remains unclear.
Poly(ADP-ribose) polymerase 1 (PARP1) is an important nuclear enzyme that regulates protein functions by poly(ADP-ribosyl)ation and gene transactivation [14]. Alternatively, PARP1 activation is required for DNA repair, but excessive activation of PARP1 leads to necrotic cell death through ATP depletion [15]. Our previous reports and those by other researchers have demonstrated that genetic and pharmacological inhibition of PARP1 is renoprotective against ischemia reperfusion injury [16], diabetes [17] and ureteral obstruction [18]. Our recent in vivo data have indicated that Parp1-deficency protects against cisplatin nephrotoxicity [19]. However, the role of PARP1 in necrosis during cisplatin nephrotoxicity and the effect of PARP1 inhibition after injury remained unclear. Therefore, we hypothesized that PARP1 activation may be required for necrosis induced by cisplatin. To investigate this hypothesis, we sought to determine whether cisplatin can induce necrosis in kidney proximal tubular cells, whether necrotic cell death is correlated with PARP1 activation, and if so, whether PARP1 inhibition post-injury can attenuate the necrotic cell death during cisplatin nephrotoxicity.
The kidney proximal tubular cell lines derived from human (HK-2) and pig (LLC-PK1) were obtained from American Type Culture Collection (Rockville, MD, USA); and the mouse proximal tubular cell line (MCT) was used as described previously [20, 21]. The HK-2, LLC-PK1, and MCT cells were maintained in Dulbecco's modified Eagle's medium (DMEM)/F-12 medium supplemented with 10% fetal bovine serum (FBS), DMEM/high-glucose supplemented with 10% FBS, and keratinocyte-serum-free medium (SFM) supplemented with 5 ng/ml human recombinant epidermal growth factor and 40 µg/ml bovine pituitary extract (Life Technologies, Grand Island, NY, USA) at 37℃ with 5% CO2, respectively. The cells were grown until 70% confluence on culture plates and then changed to SFM. After 18 hours of starvation, the cells were treated with 10, 25, 50, 100, 200, or 400 µM cisplatin (Sigma, St Louis, MO, USA) in phosphate buffered saline (control) for 0, 2, 4, or 8 hours. The cells were also treated with 1 µM (high dose) or 100 nM (low dose) PJ34 (a PARP1 inhibitor; R&D Systems, Minneapolis, MN, USA) at 2 hours after treatment with cisplatin.
The cells were stained with 1 µg/ml propidium iodide (PI; Sigma) for 10 minutes, and the percentage of PI-positive cells was assessed by fluorescence microscopy [19].
PARP activity was measured by a universal PARP assay kit (Trevigen, Gaithersburg, MD, USA).
Lactate dehydrogenase (LDH) release was measured enymatically using a CytoTox 96 Non-Radioactive Cytotoxicity Assay kit (Promega, Madison, WI, USA). Protein extracts from culture media were used to measure the levels of high mobility group box protein 1 (HMGB1) secretion using Western blot analysis [21]. Membranes were incubated with anti-HMGB1 antibody (Abcam, Cambridge, MA, USA). Peroxidase-conjugated secondary antibodies (Vector Laboratories, Burlingame, CA, USA) were applied, and a chemiluminescence reagent (PerkinElmer, Boston, MA, USA) was used to detect proteins. The bands were quantified using Lab Works analysis software (Ultra-Violet Products, Cambridge, UK).
8-Hydroxy-2'-deoxyguanosine (8-OHdG) and lipid hydroperoxide assays were performed in cell extracts using kits (Cayman, Ann Arbor, MI, USA) according to the manufacturer's instructions.
Analysis of variance was used to compare data among groups. The Pearson correlation was used for parameter correlations. All statistical analyses were performed using the Systat SigmaPlot software (Systat Software, San Jose, CA, USA). Differences between two groups were assessed by 2-tailed unpaired Student's t test. P-values less than 0.05 were considered statistically significant.
To test whether cisplatin induces necrosis in kidney proximal tubular cells, we stained with PI in kidney proximal tubular cells derived from human, pig and mouse. After treatment with cisplatin, the percentage of PI-positive cells was increased according to time and dose (Fig. 1A). Especially, treatment with high dose of cisplatin dramatically increased the percentage of PI-positive cells (Fig. 1A). Since primary necrosis is characterized by an early disruption of plasma membrane, the assessment of the plasma membrane integrity was accomplished by LDH release from human, pig and mouse proximal tubular cells. Consistent with the percentage of PI-positive cells, LDH release from kidney proximal tubular cells was also significantly increased after treatment with high dose of cisplatin (Fig. 1B). These data indicate that high dose of cisplatin induces primary necrosis in kidney proximal tubular cells.
Recently, our in vivo data have shown that PARP1 activation in mouse kidneys is exaggerated during cisplatin nephrotoxicity [19]. To determine whether treatment with cisplatin increases PARP1 activation in an in vitro model, we measured PARP activity in human, pig and mouse kidney proximal tubular cells treated with cisplatin. The level of PARP activity was not altered by treatment with low dose of cisplatin in all proximal tubular cells, but treatment with high dose of cisplatin markedly augmented the level of PARP activity (Fig. 2). As shown in Figs. 1A and 2, the level of PARP activity was significantly correlated with the percentage of PI-positive cells in all proximal tubular cells during cisplatin injury (Fig. 3). In addition, we tested whether treatment with PJ34, a potent PARP1 inhibitor, reduced the increased PARP activity after cisplatin injury. In all kidney proximal tubular cells, treatment with PJ34 at 2 hours after cisplatin injury significantly prevented the increment in PARP activity at 8 hours after the onset of injury (Fig. 4A). Furthermore, treatment with PJ34 attenuated cisplatin-induced necrosis, as demonstrated by the percentage of PI-positive cells (Fig. 4B, C). These data suggest that necrotic kidney proximal tubular cell death is dependent on PARP1 activation during cisplatin injury.
To determine whether PARP1 inhibition reduces cisplatin-induced plasma membrane disruption in kidney proximal tubular cells, we assessed the release of intracellular LDH. In human kidney proximal tubular cells, the increased percentage of LDH release after high dose of cisplatin was significantly attenuated after PARP1 inhibition in a dose-dependent manner (Fig. 5A). Since necrotic cells passively release HMGB1 chromatin protein [22], we observed the release of HMGB1 in human kidney proximal tubular cells using Western blot analysis. The endogenous HMGB1 protein was not detected in the extracellular culture media of control cells, but treatment with high dose of cisplatin led to the detection of HMGB1 in the culture media (Fig. 5B). When human kidney proximal tubular cells were treated with PJ34 at 2 hours after cisplatin injury, the amount of HMGB1 in the culture media of cisplatin-treated cells was dose-dependently decreased after PARP1 inhibition (Fig. 5B). Because excessive activation of PARP1 can mediate necrotic cell death through ATP depletion [15], we additionally measured the concentration of ATP in human kidney proximal tubular cells. Eight hours after cisplatin injury, the intracellular ATP concentration was markedly decreased; however, pharmacological inhibition of PARP significantly ameliorated the decrease in ATP level caused by cisplatin injury in a dose-dependent manner (Fig. 6). These data suggest that PARP1 is required for cisplatin-induced plasma membrane disruption and HMGB1 release.
Oxidative stress plays an integrative role between necrosis and apoptosis: severe oxidative stress induces primary necrosis and mild oxidative stress promotes early apoptosis [23], which can be mutually involved in PARP1 activation [24]. Hence, we assessed whether cisplatin-induced PARP1 activation could contribute to oxidative stress in kidney proximal tubular cells. Cisplatin injury for 6 hours dramatically increased oxidative damage to DNA, as demonstrated by the 8-OHdG level (Fig. 7A). However, the increase in oxidative damage to DNA was attenuated after PARP1 inhibition (Fig. 7A). Furthermore, cisplatin-induced lipid peroxidation demonstrated by the level of lipid hydroperoxide was consistently reduced by PARP1 inhibition (Fig. 7B). These data suggest that PARP1 activation exaggerates oxidative stress during cisplatin injury, resulting in primary necrosis.
Cisplatin-induced acute kidney injury causes renal dysfunction and tubular cell injury/death. In spite of active investigations for years, there is a gap in our understanding of the exact pathogenesis of cisplatin nephrotoxicity and cytotoxicity. Recent studies have emphasized on apoptotic cell death that seems to be the major contributor to kidney injury. Several pathways of apoptosis have been implicated, including the extrinsic pathway mediated by death receptors, the intrinsic pathway centered on mitochondria, and the endoplasmic reticulum-stress pathway [10, 25]. However, a pathway to primary necrosis during cisplatin nephrotoxicity has not been identified. The present data demonstrate novel findings that (1) cisplatin can induce primary necrosis in renal proximal tubules, (2) primary necrosis is correlated with PARP1 activation during cisplatin nephrotoxicity, and finally (3) PARP1 inhibition after injury attenuates kidney proximal tubular necrosis during cisplatin nephrotoxicity. The data suggest that necrosis can be a major determinant of cisplatin nephrotoxicity; thus, supporting the finding that the predominant lesion consists of acute necrosis in patients with cisplatin-induced acute kidney injury [26].
Necrotic and apoptotic cell death in kidney tubular epithelium are the main histopathological characteristic in an in vivo cisplatin nephrotoxicity model [10]. Especially, the proximal tubule is the major site of cell death induced by cisplatin [27]. Apoptosis occurs upon low concentrations of cisplatin for a long time, whereas necrosis is observed at high concentrations of cisplatin for a short time [9]. In the previous study, we have demonstrated that Parp1 deficiency prevents tubular necrosis induced by cisplatin in vivo [19]. This finding is consistent with the present results in kidney proximal tubular cells, suggesting that PARP1-dependent kidney proximal tubular necrosis is a principal factor in cisplatin nephrotoxicity and causes subsequent kidney dysfunction. Although some of the earlier reports have suggested the involvement of PARP1 in the apoptotic pathway [28, 29], the majority of other and our studies has provided evidence that PARP1 activation induces necrosis in various cell/tissue injury models [15, 18, 19, 30].
Necrotic tubules can release alarming factors such as HMGB1 to activate an inflammatory response [31]. HMGB1 is a nuclear factor that is involved in transcriptional activation and DNA folding [32], and when it is released into the extracellular space as a cytokine or ligand to trigger toll-like receptors' signaling, it can be a critical mediator of innate immune responses to injury and infection [33]. Although HMGB1 does not plays a significant role in cellular responses to cisplatin [34], HMGB1 released from necrotic cells during cisplatin injury may contribute to inflammatory responses. The cisplatin-induced inflammatory response results in the development of kidney tissue damage and kidney dysfunction, which cause acute kidney injury [35, 36]. In ischemic acute kidney injury, the released HMGB1 acts as a mediator of kidney damage through the toll-like receptor 4 pathway [37]; and PARP1 activation induces HMGB1 release from mouse embryonic fibroblasts [38]. In our previous study, we have shown that Parp1 deficiency reduces kidney inflammation during cisplatin nephrotoxicity, as well as tubular necrosis [19]. Consistent with the previous reports, our present data show for the first time that PARP1 inhibition after injury results in reduced release of HMGB1 from kidney proximal tubular cells treated with cisplatin, suggesting that PARP1-dependent HMGB1 release from kidney proximal tubular cells contributes to inflammation during cisplatin nephrotoxicity.
During cisplatin nephrotoxicity, necrosis may not be exclusively due to ATP depletion as our data demonstrate increased oxidative stress in cisplatin-treated kidney proximal tubular cells using 8-OHdG and lipid hydroperoxide assays, and reduced oxidative stress after PARP1 inhibition after injury. Mitochondrial complex I produces reactive oxygen species through excessive activation of PARP1 after ischemia and reperfusion injury [24], and poly (ADP-ribosyl)ated mitochondrial proteins including components of the electron transport chain can further increase reactive oxygen species production [39, 40]. Previous studies have demonstrated that excessive oxidative stress damages DNA and further induces PARP1 activation leading to a positive feedback mechanism that exacerbates necrotic cell death, which is consistent with our present data in kidney proximal tubular cells.
Taken together, this study shows that cisplatin increases PARP1 activation in kidney proximal tubular cells, and further, the increased PARP1 activation contributes to proximal tubular necrosis through oxidative stress. PARP1 may be a pivotal molecule involved in cisplatin-mediated proximal tubular necrosis.
Figures and Tables
 | Fig. 1Cisplatin induces necrosis in kidney proximal tubular cells. After 18 hours of starvation, HK-2, LLC-PK1, and MCT cells were treated with 10, 25, 50, 100, 200, or 400 µM of cisplatin in phosphate buffered saline (control) for 0, 2, 4, or 8 hours. (A) The percentage of propidium iodide (PI)-positive cells was assessed in 10 field (×400) per well. (B) Lactate dehydrogenase (LDH) release was measured enzymatically using a CytoTox 96 Non-Radioactive Cytotoxicity Assay kit (Promega). Error bars represent SD (n=3 experiments). *P<0.05, **P<0.01, ***P<0.001 versus 0 hour. |
 | Fig. 2Cisplatin increases poly(ADP-ribose) polymerase (PARP) activity in kidney proximal tubular cells. After 18 hours of starvation, HK-2, LLC-PK1, and MCT cells were treated with 10, 25, 50, 100, 200, or 400 µM of cisplatin in phosphate buffered saline (control) for 0, 2, 4, or 8 hours. PARP activity was measured by a universal PARP assay kit (Trevigen). Error bars represent SD (n=3 experiments). *P<0.05, **P<0.01, ***P<0.001 versus 0 hour. |
 | Fig. 3Correlation between the percentage of propidium iodide (PI)-positive cells and poly(ADP-ribose) polymerase (PARP) activity in kidney proximal tubular cells during cisplatin injury. After 18 hours of starvation, HK-2, LLC-PK1, and MCT cells were treated with 10, 25, 50, 100, 200, or 400 µM of cisplatin in phosphate buffered saline (control) for 0, 2, 4, or 8 hours. The Pearson correlation between the percentage of PI-positive cells and the level of PARP activity at all cisplatin-dose levels and time points shown in Figs. 1A and 2. |
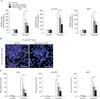 | Fig. 4Poly(ADP-ribose) polymerase 1 (PARP1) inhibition reduces necrosis induced by cisplatin in kidney proximal tubular cells after cisplatin injury. After 18 hours of starvation, HK-2, LLC-PK1, and MCT cells were treated with 400 µM cisplatin for 8 hours. The cells were also treated with high or low dose of PJ34 at 2 hours after treatment with cisplatin. (A) PARP activity was measured by a universal PARP assay kit. (B) Necrosis in HK-2 cells was detected by propidium iodid (PI) staining. Nuclei were counterstained with DAPI. Scale bars=50 µm. (C) The percentage of PI-positive cells was assessed in 10 fields (×400) per well. Error bars represent SD (n=3 experiments). **P<0.01 versus control. †P<0.05, ††P<0.01, †††P<0.001 versus vehicle. |
 | Fig. 5Poly(ADP-ribose) polymerase 1 (PARP1) inhibition reduces plasma membrane disruption and HMGB1 release induced by cisplatin in kidney proximal tubular cells. After 18 hours of starvation, HK-2 cells were treated with 400 µM cisplatin for 8 hours. The cells were also treated with high or low dose of PJ34 at 2 hours after treatment with cisplatin. After 8 hours of cisplatin injury, the cells and media were harvested. (A) Lactate dehydrogenase (LDH) release was measured enzymatically using a CytoTox 96 Non-Radioactive Cytotoxicity Assay kit. (B) The levels of released HMGB1 in cell culture media were measured using Western blot analysis. Protein bands were quantified using Lab Works analysis software (Ultra-Violet Products). Error bars represent SD (n=3 experiments). **P<0.01 versus control. †P<0.05, ††P<0.01, †††P<0.001 versus vehicle. |
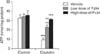 | Fig. 6Poly(ADP-ribose) polymerase 1 (PARP1) inhibition ameliorates ATP depletion induced by cisplatin in kidney proximal tubular cells. After 18 hours of starvation, HK-2 cells were treated with 400 µM cisplatin for 8 hours. The cells were also treated with high or low dose of PJ34 at 2 hours after treatment with cisplatin. The level of ATP was measured by an ATP assay kit (BioVision). Error bars represent SD (n=3 experiments). **P<0.01, ***P<0.001 versus control. ††P<0.01, †††P<0.001 versus vehicle. |
 | Fig. 7Poly(ADP-ribose) polymerase 1 (PARP1) inhibition reduces oxidative stress induced by cisplatin in kidney proximal tubular cells. After 18 hours of starvation, HK-2 cells were treated with 400 µM cisplatin for 8 hours. The cells were also treated with high or low dose of PJ34 at 2 hours after treatment with cisplatin. The levels of 8-OHdG (A) and lipid hydroperoxide (B) were measured by assay kits (Cayman). Error bars represent SD (n=3 experiments). **P<0.01 versus control. †P<0.05, ††P<0.01, †††P<0.001 versus vehicle. |
References
1. Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003; 22:7265–7279.
2. Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov. 2005; 4:307–320.
3. Stark JJ, Howel SB. Nephrotoxicity of cis-platinum (II) dichlorodiammine. Clin Pharmacol Ther. 1978; 23:461–466.
4. Arany I, Safirstein RL. Cisplatin nephrotoxicity. Semin Nephrol. 2003; 23:460–464.
5. Megyesi J, Safirstein RL, Price PM. Induction of p21WAF1/CIP1/SDI1 in kidney tubule cells affects the course of cisplatin-induced acute renal failure. J Clin Invest. 1998; 101:777–782.
6. Li S, Basnakian A, Bhatt R, Megyesi J, Gokden N, Shah SV, Portilla D. PPAR-alpha ligand ameliorates acute renal failure by reducing cisplatin-induced increased expression of renal endonuclease G. Am J Physiol Renal Physiol. 2004; 287:F990–F998.
7. Wei Q, Dong G, Franklin J, Dong Z. The pathological role of Bax in cisplatin nephrotoxicity. Kidney Int. 2007; 72:53–62.
8. Pabla N, Murphy RF, Liu K, Dong Z. The copper transporter Ctr1 contributes to cisplatin uptake by renal tubular cells during cisplatin nephrotoxicity. Am J Physiol Renal Physiol. 2009; 296:F505–F511.
9. Lieberthal W, Triaca V, Levine J. Mechanisms of death induced by cisplatin in proximal tubular epithelial cells: apoptosis vs. necrosis. Am J Physiol. 1996; 270(4 Pt 2):F700–F708.
10. Pabla N, Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int. 2008; 73:994–1007.
11. Pabla N, Huang S, Mi QS, Daniel R, Dong Z. ATR-Chk2 signaling in p53 activation and DNA damage response during cisplatin-induced apoptosis. J Biol Chem. 2008; 283:6572–6583.
12. Dong G, Luo J, Kumar V, Dong Z. Inhibitors of histone deacetylases suppress cisplatin-induced p53 activation and apoptosis in renal tubular cells. Am J Physiol Renal Physiol. 2010; 298:F293–F300.
13. Yang C, Kaushal V, Shah SV, Kaushal GP. Autophagy is associated with apoptosis in cisplatin injury to renal tubular epithelial cells. Am J Physiol Renal Physiol. 2008; 294:F777–F787.
14. Kraus WL, Lis JT. PARP goes transcription. Cell. 2003; 113:677–683.
15. Ha HC, Snyder SH. Poly(ADP-ribose) polymerase is a mediator of necrotic cell death by ATP depletion. Proc Natl Acad Sci U S A. 1999; 96:13978–13982.
16. Martin DR, Lewington AJ, Hammerman MR, Padanilam BJ. Inhibition of poly(ADP-ribose) polymerase attenuates ischemic renal injury in rats. Am J Physiol Regul Integr Comp Physiol. 2000; 279:R1834–R1840.
17. Shevalye H, Stavniichuk R, Xu W, Zhang J, Lupachyk S, Maksimchyk Y, Drel VR, Floyd EZ, Slusher B, Obrosova IG. Poly(ADP-ribose) polymerase (PARP) inhibition counteracts multiple manifestations of kidney disease in long-term streptozotocin-diabetic rat model. Biochem Pharmacol. 2010; 79:1007–1014.
18. Kim J, Padanilam BJ. Loss of poly(ADP-ribose) polymerase 1 attenuates renal fibrosis and inflammation during unilateral ureteral obstruction. Am J Physiol Renal Physiol. 2011; 301:F450–F459.
19. Kim J, Long KE, Tang K, Padanilam BJ. Poly(ADP-ribose) polymerase 1 activation is required for cisplatin nephrotoxicity. Kidney Int. 2012; 82:193–203.
20. Haverty TP, Kelly CJ, Hines WH, Amenta PS, Watanabe M, Harper RA, Kefalides NA, Neilson EG. Characterization of a renal tubular epithelial cell line which secretes the autologous target antigen of autoimmune experimental interstitial nephritis. J Cell Biol. 1988; 107:1359–1368.
21. Kim J, Padanilam BJ. Renal nerves drive interstitial fibrogenesis in obstructive nephropathy. J Am Soc Nephrol. 2013; 24:229–242.
22. Rovere-Querini P, Capobianco A, Scaffidi P, Valentinis B, Catalanotti F, Giazzon M, Dumitriu IE, Müller S, Iannacone M, Traversari C, Bianchi ME, Manfredi AA. HMGB1 is an endogenous immune adjuvant released by necrotic cells. EMBO Rep. 2004; 5:825–830.
23. Fleury C, Mignotte B, Vayssière JL. Mitochondrial reactive oxygen species in cell death signaling. Biochimie. 2002; 84:131–141.
24. Zhou HZ, Swanson RA, Simonis U, Ma X, Cecchini G, Gray MO. Poly(ADP-ribose) polymerase-1 hyperactivation and impairment of mitochondrial respiratory chain complex I function in reperfused mouse hearts. Am J Physiol Heart Circ Physiol. 2006; 291:H714–H723.
25. Kim N, Choi WS. Proapoptotic role of nuclear clusterin in brain. Anat Cell Biol. 2011; 44:169–175.
26. Yao X, Panichpisal K, Kurtzman N, Nugent K. Cisplatin nephrotoxicity: a review. Am J Med Sci. 2007; 334:115–124.
27. Shino Y, Itoh Y, Kubota T, Yano T, Sendo T, Oishi R. Role of poly(ADP-ribose)polymerase in cisplatin-induced injury in LLC-PK1 cells. Free Radic Biol Med. 2003; 35:966–977.
28. Simbulan-Rosenthal CM, Rosenthal DS, Iyer S, Boulares H, Smulson ME. Involvement of PARP and poly(ADP-ribosyl)ation in the early stages of apoptosis and DNA replication. Mol Cell Biochem. 1999; 193:137–148.
29. Oliver FJ, de la Rubia G, Rolli V, Ruiz-Ruiz MC, de Murcia G, Murcia JM. Importance of poly(ADP-ribose) polymerase and its cleavage in apoptosis. Lesson from an uncleavable mutant. J Biol Chem. 1998; 273:33533–33539.
30. Filipovic DM, Meng X, Reeves WB. Inhibition of PARP prevents oxidant-induced necrosis but not apoptosis in LLC-PK1 cells. Am J Physiol. 1999; 277:F428–F436.
31. Goligorsky MS. TLR4 and HMGB1: partners in crime? Kidney Int. 2011; 80:450–452.
32. Javaherian K, Liu JF, Wang JC. Nonhistone proteins HMG1 and HMG2 change the DNA helical structure. Science. 1978; 199:1345–1346.
33. Yang H, Wang H, Czura CJ, Tracey KJ. The cytokine activity of HMGB1. J Leukoc Biol. 2005; 78:1–8.
34. Wei M, Burenkova O, Lippard SJ. Cisplatin sensitivity in Hmbg1-/- and Hmbg1+/+ mouse cells. J Biol Chem. 2003; 278:1769–1773.
35. Zhang B, Ramesh G, Uematsu S, Akira S, Reeves WB. TLR4 signaling mediates inflammation and tissue injury in nephrotoxicity. J Am Soc Nephrol. 2008; 19:923–932.
36. Kim J, Kim JI, Na YK, Park KM. Intra-renal slow cell-cycle cells contribute to the restoration of kidney tubules injured by ischemia/reperfusion. Anat Cell Biol. 2011; 44:186–193.
37. Wu H, Ma J, Wang P, Corpuz TM, Panchapakesan U, Wyburn KR, Chadban SJ. HMGB1 contributes to kidney ischemia reperfusion injury. J Am Soc Nephrol. 2010; 21:1878–1890.
38. Ditsworth D, Zong WX, Thompson CB. Activation of poly (ADP)-ribose polymerase (PARP-1) induces release of the pro-inflammatory mediator HMGB1 from the nucleus. J Biol Chem. 2007; 282:17845–17854.
39. Lai Y, Chen Y, Watkins SC, Nathaniel PD, Guo F, Kochanek PM, Jenkins LW, Szabó C, Clark RS. Identification of poly-ADP-ribosylated mitochondrial proteins after traumatic brain injury. J Neurochem. 2008; 104:1700–1711.
40. Pankotai E, Lacza Z, Murányi M, Szabó C. Intra-mitochondrial poly(ADP-ribosyl)ation: potential role for alpha-ketoglutarate dehydrogenase. Mitochondrion. 2009; 9:159–164.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download