Abstract
Sirtuins (SIRTs) are involved in multiple cellular processes including those related to aging, cancer, and a variety of cellular functions including cell cycle progression, DNA repair, and cellular proliferation. SIRTs have been shown to extend the yeast life span, although there is presently little known about SIRT expression in the organs of mice. In the present study, we were especially interested in identifying differences in SIRT expression between young mice and aged mice. Specifically, we investigated the expression of SIRT1 and SIRT3 in the kidney, lung, skin, adipose tissue, and spleens of 6-month-old and 24-month-old mice using immunohistochemical staining. Compared with that in younger mice, the expression of SIRT1 in 24-month-old rats was increased in kidney, lung, and spleen tissue, while that of SIRT3 was decreased in adipose, kidney, and lung tissue. The results of our study suggest that aging is associated with altered patterns of expression of SIRT1 and SIRT3. In addition, we noted that the expression patterns of SIRT1 and SIRT3 varied by organ. Taken together, the results of this study suggest the possibility that SIRTs may be involved in diseases associated with aging.
Rats fed a calorie-restricted diet have been shown to live longer than freely fed controls; however, the underlying molecular mechanism for this extension of life span by a calorie-restricted diet remains unclear [1]. First described as a silencing factor in yeast (silencing information regulator, SIR), Sirt2 has been recognized as a modulator of the yeast life span [2]. Indeed, the seven mammalian sirtuins, sirtuin 1 (SIRT1) to sirtuin 7 (SIRT7), have been shown to act as determinants of life span in yeast mother cells. Likewise, the mammalian sirtuin proteins (homologues of the yeast sirtuin SIRT2) have received much attention for their role in regulatory processes, primarily metabolism and aging [3].
Sirtuins are a family of NAD(+)-dependent enzymes that share a primarily protective function in the development of many age-related diseases, including cancer, neurodegeneration, and cardiovascular disease [1]. Sirtuins are found in different subcellular locations, including the nucleus, cytosol, and mitochondria. The regulation of mammalian lifespan by sirtuins has important therapeutic implications for age-related diseases. In yeast, Sirt2, Sirt3, and Sirt4 mutants have a short life span, which is caused indirectly by an increase in rDNA recombination [2].
Sirt3 is a mitochondrial deacetylase and an essential player in enhancement of the mitochondrial glutathione antioxidant defense system during caloric restriction (CR) diet, suggesting that Sirt3-dependent mitochondrial adaptations may be a central mechanism of aging retardation in mammals [4]. Indeed, the protective effects of CR diet on oxidative stress and damage are diminished in mice lacking SIRT3 [5, 6]. In addition, SIRT3 is essential to the calorie restriction-mediated reduction of oxidative damage in multiple tissue types [4]. SIRT3 has been shown to directly modulate levels of reactive oxygen species (ROS), potentially suggesting a role for SIRT3 in regulating age-related pathologies that depend on cellular levels of ROS [6].
SIRTs may control cellular metabolism and homeostasis. Specifically, SIRTs help cells to adapt to insufficient uptake of nutrients. Further, the metabolism of cancer cells is adapted to facilitate the uptake and incorporation of nutrients into the biomass (e.g., nucleotides, amino acids, and lipids) needed to produce new cells [7]. In this way, SIRTs may be important in cancer metabolism and genetic stabilization, which in turn may play a role in tumorigenesis. Consistent with this possibility, SIRT6 has been shown to act as potent tumor suppressor by suppressing metabolism in cancer cells [8].
SIRTs are famous of their anti-aging action. But there are no reports whether their expression levels modulate by aging. Moreover there are a little reports focus on the change of their expression levels in organs, correlated by aging. In this study, we demonstrated that SIRT1 and SIRT3 exhibit age- and organ-specific patterns of expression by evaluating the skin, kidney, lung, and spleen of 24-month-old mice compared to relatively younger (6-month-old) mice. We also discuss the importance of our findings in the context of age-related disease and the aging process.
Six male mice aged 6 months were used in this study. The mice were adapted to the animal laboratory environment for 3 days, during which they were housed 3 per windowless Plexiglas cage at 22℃ in the Animal Laboratory of the College of Medicine of Kosin University. The animals were then sacrificed. Animal experiments were conducted within the guidelines for animal experiment established by the Animal Laboratory of the College of Medicine of Kosin University and with their approval. Tissues of 24-month-old mice were obtained from the Aging Tissue Bank (Busan, Korea).
Mice were anesthetized and perfused transcardially with cold phosphate buffered saline (PBS), followed by 4% paraformaldehyde. Skin, spleen, kidney, lung, liver, and adipose tissues were immediately removed and fixed overnight. The samples were then washed in 70% ethanol to remove the fixative. The tissue samples were dehydrated in ethanol, cleared in xylene and embedded in paraffin using standard protocols. We subsequently obtained paraffin-embedded histological sections at a thickness of 5 µm.
Antigen labeling was performed using an avidin-biotin complex technique. Sections were processed for immunohistochemistry with SIRT1 and SIRT3 antibodies using standard techniques. Briefly, sections were rinsed with PBS for 30 minutes and incubated in a normal blocking solution of PBS with 10% normal goat serum and 3% Triton X-100 for 1 hour and polyclonal anti-SIRT1 (1:200, Santa Cruz Co., Santa Cruz, CA, USA) or polyclonal anti-SIRT3 (1:200, Cell Signaling Co., Beverly, MA, USA) overnight at 4℃. After washing in PBS, sections were incubated with biotin-conjugated goat anti-rabbit IgG (1:200, Vector Lab, Burlingame, CA, USA) and then incubated in avidin-biotin complex solution (Vector Lab) for 1 hour at room temperature. Finally, sections were placed in 3,3'-diaminobenzidine tetrahydrochloride (DAB) and viewed and photographed with a light microscope (Nikon Eclipse 80i, Nikon, Tokyo, Japan).
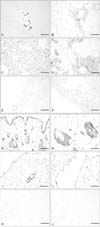
To determine the pattern of expression of SIRT1, we performed immunohistochemical staining of various organs including adipose tissue, kidney, spleen, skin, lung, and liver (Fig. 1). In adipose tissue, expression of SIRT1 was not detected in both 6-month-old and 24-month-old mice (Fig. 1A, B). The expression of SIRT1 was decreased in the kidney of 24-month-old mice compared with that of younger 6-month-old mice (Fig. 1C, D). In addition, we found that SIRT1 was localized in the nucleus of renal tubular cells in 6-month-old mice. In the spleen, SIRT1 was expressed only in a few immune cells (Fig. 1E, F), and there was little difference in expression with age. In skin and subcutaneous tissue, SIRT1 was weakly expressed in both young and old mice (Fig. 1G, H). SIRT1 was localized in the nuclei of epithelial cells of the sebaceous glands and was also present in the nuclei of epidermal cells. Expression of SIRT1 in the skin showed some difference with age. SIRT1 was not expressed in the lungs of 6-month-old mice, whereas it was expressed in the lungs of 24-month-old mice, including some alveolar epithelial cells and mesothelial cells of the visceral pleura covering the lung surface (Fig. 1I, J). Lastly, SIRT1 was not expressed in the liver of either young or old mice (Table 1, Fig. 1K, L).
To examine the expression of SIRT3 protein, we performed immunohistochemical staining of various organs including adipose tissue, kidney, spleen, skin, lung, and liver (Fig. 2). In adipose tissue, expression of SIRT3 was decreased in 24-month-old mice (Fig. 2A, B). Some connective tissue cells were prominently labeled by the SIRT3 antibody in 6-month-old mice; however, SIRT3 was not expressed in adipose cells nor connective tissue cells of 24-month-old mice.
Expression of SIRT3 was decreased in kidney tissue of 24-month-old mice compared to that in 6-month-old mice (Fig. 2C, D). In addition, SIRT3 was expressed in renal cortical tubular cells of younger mice, while the numbers of SIRT3-positive tubular cells was decreased in older mice. SIRT3 was expressed in immune cells present in the spleens of mice at both ages but exhibited increased staining in 24-month-old mice (Fig. 2E, F).
We determined that SIRT3 was expressed in epidermal cells and sebaceous glandular cells, especially in the nucleus. In subcutaneous tissue, SIRT3 was expressed in some fibroblasts, although there was no age-dependent change in SIRT3 expression (Fig. 2G, H). Immunohistochemical staining of lung sections revealed that SIRT3 expression was increased in 24-month-old mice (Fig. 2I, J) and was prominent not only in epithelial cells, smooth muscle cells, and connective tissue cells of the bronchioles, but also alveolar cells. Lastly, SIRT3 was not expressed in the livers of either young or old mice (Table 2, Fig. 2K, L).
Taken together, our results suggest that both SIRT1 and SIRT3 are expressed in various organs including the lung, kidney, adipose tissue, spleen, and skin but not in the liver.
To determine whether expression of sirtuin proteins changes with age, we performed immunohistochemical staining for SIRT1 and SIRT3 in 6-month-old and 24-month-old mice. Our results showed that, in kidney tissue of 24-month-old mice, expression of SIRT1 was decreased slightly compared to that in 6-month-old mice. Specifically, SIRT1 was prominently expressed in the nucleus and cytoplasm of renal tubular cells in 6-month-old mice. We also found that SIRT1 expression was increased slightly in the lung and skin of older mice compared with younger mice. However, we found no evidence of SIRT1 expression in hepatocytes of either young or old mice. Importantly, expression of SIRT1 was found to vary with age.
In addition to SIRT1, we found that expression of SIRT3 was decreased in kidney epithelial cells of 6-month-old mice. Specifically, SIRT3 was expressed prominently in renal cortical tubular cells of younger mice. In the lung, nuclear localization of SIRT3 was obvious in alveolar cells, smooth muscle cells, and connective tissue cells of bronchioles and was generally increased in the lungs of 24-month-old mice compared with younger mice. We also determined that SIRT3 was expressed in epidermal cells and sebaceous glandular cells, especially the nuclei. In subcutaneous tissue, we showed that fibroblasts expressed SIRT3. In aged mice, expression of SIRT3 was increased in the skin, although the difference between young and old mice was not dramatic.
To date, there have been no reports of organ-specific expression of SIRT1 and SIRT3 in mammals. Moreover, there have been no basic studies comparing the expression of SIRT1 and SIRT3 as a function of aging. In cultured lung cancer cells, SIRT1 can repress programmed cell death and senescence to alter DNA repair, stress response and aging [9]. Thus, SIRT1 may have both pro- and anti-carcinogenic properties. Like SIRT1, SIRT3 is a member of the mammalian sirtuin family of proteins and is expressed in human lung adenocarcinoma cells. The expression of SIRT3 is decreased in human lung adenocarcinoma tissue compared with normal adjacent tissue [10]. In addition, the expression of SIRT1 in normal lung tissue and its specific role in vivo were previously unknown. In the present study, we determined that SIRT3 expression was decreased slightly in alveolar and bronchiolar epithelial cells of older mice compared with those in younger mice. In general, our data also suggest that the expression of SIRT3 in organs varies with age.
It has been previously reported in a model of kidney ischemia that younger mice express higher levels of SIRT1 compared with older mice [11]. On the contrary, our findings showed that the number of renal tubular cells that exhibited positive expression of SIRT1 increased with age in normal kidney tissue. Kidney ischemia-reperfusion injury induces cellular injury in the proximal tubule, which may include mitochondrial dysfunction. SIRT1 acts to facilitate restoration of mitochondrial proteins including ATP synthase beta and COX I in proximal tubular cells [12]. Thus, in the kidneys, SIRT1 may repress renal cell apoptosis and fibrosis and regulate lipid metabolism, autophagy, blood pressure, and sodium balance [13]. Expression of SIRT1 was detected only in some proximal tubular cells of 24-month-old mice, which may reflect an association with the functional status of these tubular cells. Our immunohistochemical staining results also showed that SIRT3 was expressed in renal cortical tubular cells, while the number of SIRT3-positive cells was decreased in older mice. In addition, we found that SIRT3 was present in both the nucleus and cytoplasm and exhibited the strongest intensity in the kidney compared with other organs. However, despite these findings, the functions of SIRT3 in the kidney remain elusive.
With respect to the spleen, there was no evidence of SIRT1 expression; however, SIRT3 exhibited a broad pattern of cellular localization in the immune cells of the spleen. In addition, the expression of SIRT3 increased with age in the spleen. Although we did not observe expression of SIRT1 in the spleen, it is known as a critical regulator of both the innate and adaptive immune responses in mice, and its altered functions are likely involved in autoimmune diseases [14].
It has been reported that subcutaneous adipose tissue expresses both SIRT1 and SIRT3 mRNA. Weight loss induces expression of SIRT1 and SIRT3 in subcutaneous adipose tissue, which may reflect the involvement of these proteins in metabolic and inflammatory pathways and potentially explain their role in improving health and increasing lifespan [15]. In the present study, we showed that subcutaneous adipose tissue expressed both SIRT1 and SIRT3. Interestingly, expression of Sirt1 was decreased with age and was localized exclusively in adipose cells. On the other hand, SIRT3 was not expressed in adipose cells but was present in fibroblasts associated with adipose tissue. Furthermore, the staining intensity of fibroblasts with SIRT3 decreased with age.
SIRT1 is expressed in cultured human skin keratinocytes [16]. Our data show that keratinocytes expressed low levels of SIRT1. Interestingly, keratinocytes of 6-month-old mice expressed higher levels of SIRT3 compared with older mice, which were negative for SIRT3 staining in keratinocytes. Glandular cells and hair follicle cells intensely express SIRT3. Further, SIRT3 expression was not diminished with age.
Our immunohistochemical study indicates that aging is correlated with altered patterns of expression of both SIRT1 and SIRT3, and that the expression of these two proteins varies among tissue of different organs. At present, the reasons for the differential patterns of expression of SIRT1 and SIRT3 by age and tissue type are unclear. Likewise, the specific functions of members of the SIRT family remain controversial. According to some studies, SIRT proteins may have anti-cancer functions, whereas other researchers have shown that they repress apoptosis and have pro-cancer functions. SIRT proteins are famous for their role in extending the life of yeast cells. If applicable to mammals, SIRTs have been proposed to be involved in diseases associated with aging. In the future, we will examine whether SIRT proteins protect against age-related diseases in mammals and investigate how they function in life extension.
Figures and Tables
Fig. 1
Representative immunohistochemical micrographs of adipose tissue (A, B), kidney (C, D), spleen (E, F), skin (G, H), lung (I, J), and liver (K, L). Immunoreactivity of SIRT1 in 6-month-old rat (left column) as compared of 2-year-old rat (right column). Scale bars=100 µm (A-L).

Fig. 2
Representative immunohistochemical micrographs of adipose tissue (A, B), kidney (C, D), spleen (E, F), skin (G, H), lung (I, J), and liver (K, L). Immunoreactivity of SIRT3 in 6-month-old rat (left column) as compared of 2-year-old rat (right column). Scale bars=100 µm (A-L).
Acknowledgements
This work was supported by Research Grant from the Kosin University, College of Medicine.
References
1. Sebastian C, Satterstrom FK, Haigis MC, Mostoslavsky R. From sirtuin biology to human diseases: an update. J Biol Chem. 2012; 287:42444–42452.
2. Kaeberlein M, McVey M, Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999; 13:2570–2580.
3. Houtkooper RH, Pirinen E, Auwerx J. Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol. 2012; 13:225–238.
4. Someya S, Yu W, Hallows WC, Xu J, Vann JM, Leeuwenburgh C, Tanokura M, Denu JM, Prolla TA. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell. 2010; 143:802–812.
5. Qiu X, Brown K, Hirschey MD, Verdin E, Chen D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab. 2010; 12:662–667.
6. Tao R, Coleman MC, Pennington JD, Ozden O, Park SH, Jiang H, Kim HS, Flynn CR, Hill S, Hayes McDonald W, Olivier AK, Spitz DR, Gius D. Sirt3-mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress. Mol Cell. 2010; 40:893–904.
7. Kanfi Y, Naiman S, Amir G, Peshti V, Zinman G, Nahum L, Bar-Joseph Z, Cohen HY. The sirtuin SIRT6 regulates lifespan in male mice. Nature. 2012; 483:218–221.
8. Sebastian C, Zwaans BM, Silberman DM, Gymrek M, Goren A, Zhong L, Ram O, Truelove J, Guimaraes AR, Toiber D, Cosentino C, Greenson JK, MacDonald AI, McGlynn L, Maxwell F, Edwards J, Giacosa S, Guccione E, Weissleder R, Bernstein BE, Regev A, Shiels PG, Lombard DB, Mostoslavsky R. The histone deacetylase SIRT6 is a tumor suppressor that controls cancer metabolism. Cell. 2012; 151:1185–1199.
9. Chen X, Hokka D, Maniwa Y, Ohbayashi C, Itoh T, Hayashi Y. Sirt1 is a tumor promoter in lung adenocarcinoma. Oncol Lett. 2014; 8:387–393.
10. Xiao K, Jiang J, Wang W, Cao S, Zhu L, Zeng H, Ouyang R, Zhou R, Chen P. Sirt3 is a tumor suppressor in lung adenocarcinoma cells. Oncol Rep. 2013; 30:1323–1328.
11. Fan H, Yang HC, You L, Wang YY, He WJ, Hao CM. The histone deacetylase, SIRT1, contributes to the resistance of young mice to ischemia/reperfusion-induced acute kidney injury. Kidney Int. 2013; 83:404–413.
12. Funk JA, Schnellmann RG. Accelerated recovery of renal mitochondrial and tubule homeostasis with SIRT1/PGC-1alpha activation following ischemia-reperfusion injury. Toxicol Appl Pharmacol. 2013; 273:345–354.
13. Kitada M, Kume S, Takeda-Watanabe A, Kanasaki K, Koya D. Sirtuins and renal diseases: relationship with aging and diabetic nephropathy. Clin Sci (Lond). 2013; 124:153–164.
14. Kong S, McBurney MW, Fang D. Sirtuin 1 in immune regulation and autoimmunity. Immunol Cell Biol. 2012; 90:6–13.
15. Moschen AR, Wieser V, Gerner RR, Bichler A, Enrich B, Moser P, Ebenbichler CF, Kaser S, Tilg H. Adipose tissue and liver expression of SIRT1, 3, and 6 increase after extensive weight loss in morbid obesity. J Hepatol. 2013; 59:1315–1322.
16. Cao C, Lu S, Kivlin R, Wallin B, Card E, Bagdasarian A, Tamakloe T, Wang WJ, Song X, Chu WM, Kouttab N, Xu A, Wan Y. SIRT1 confers protection against UVB- and H2O2-induced cell death via modulation of p53 and JNK in cultured skin keratinocytes. J Cell Mol Med. 2009; 13:3632–3643.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download