Abstract
Tissue engineering is a new field of which the main purpose is to regenerate and repair the damaged tissues. Scaffolds serve as three dimensional matrices for neo-organogenesis and their substance can be biologic or synthetic. Natural polymers have good interactions with the cells and synthetic biomaterials are also highly useful in biomedical application because of their biocompatible properties. In addition to scaffold substance, surface properties of biomaterials have an important role in tissue engineering. In this study, we examined whether substrate substance is important for wound healing or its surface topography. Therefore, we fabricated two matrices, electrospun polycaprolactone (PCL) nanofibers and collagen/chitosan film, and implanted them to the same rat models. After 2 weeks, the sizes of healing wounds were measured and their cellular structures were evaluated by histochemistry and mmunohistochemistry. Histological staining showed a good level of cellularization and epidermis-dermis formation in PCL implant while no determinable epithelium was observed after 2 weeks in collagen-chitosan graft. Immunohistochemical study demonstrated the highly expressed pancytokeratin in PCL graft while its expression was weak in underdeveloped epidermis of collagen-chitosan implantation. In conclusion, this study suggested that PCL nanofibers with high surface area had a more ideal property than natural collagen-chitosan film, therefore the structure and topography of a matrix seemed to be more important in wound healing than its material substance.
Tissue engineering is a new interdisciplinary field in which the engineering sciences and biology are incorporated to regenerate the damaged tissues and replace them by new ones. The cells, the scaffold type and the suitable conditions conducive to cells proliferation and differentiation are the essential factors in tissue engineering [1]. Scaffolds as three dimensional (3D) matrices inevitably influence cellular infiltration, proliferation, differentiation and neo-organogenesis [23]. According to Jayarama et al. [4], scaffolds must have some essential properties to be used in tissue engineering: they must be biomimetic, physically stable for implantation, physiologically active to effectively control repair and regeneration, biodegradable after in vivo implantation, and should not be toxic for cells to replacement or repair of the original tissue or organ. It should be emphasized on this point that the scaffold substance and its manufacture technologies could play a crucial role in tissue engineering. Both biologic and synthetic materials can be used to fabricate 3D scaffolds. Natural polymers have better interactions with the cells and allow them to enhance performance in a biological system. Besides, synthetic biomaterials are highly useful in biomedical application because of their properties (e.g., porosity, degradation time, and mechanical characteristics) [5].
In addition to scaffold substance, surface morphology of a matrix can play an important role in tissue engineering. Many studies have shown that cells cultured on scaffolds with different surface properties, including surface chemistry, geometry and topography, exhibit a wide range of behaviors [2678910]. Moreover, mechanical strength and topography of 3D scaffolds have been indicated to be effective on cellular activities such as cell migration and morphology in tissue engineering [911]. Besides, it has been suggested that cell behaviors in a 3D scaffold can differ from those on flat surfaces and that the 3D scaffolds are suitable for long-lasting cell culture because of their high specific surface area [12]. However, it was shown that cells proliferate slowly in 3D fibrous scaffolds as compared to those cultured on flat surface because fewer cells are directly attached to the fiber surfaces [13]. Up to now, many biomimetic scaffolds have been fabricated for skin tissue engineering using polymers with various degrees of strength in sponge-, fibrous-, or gel-type forms [4]. Nanofibrous polycaprolactone (PCL) is a reliable substrate for supporting the growth and differentiation of a variety of cell types and abundantly applied for skin [4]. PCL is a biocompatible and biodegradable synthetic polymer with good mechanical properties [1415] that has been electrospun easily [12345678910111213141516]. However, it is noteworthy that this polymer is hydrophobic, has very few cell recognition sites and degrades slowly [6]. On the other side, natural polymers are commonly utilized because of their enhanced biocompatibility and biofunctional motifs [17]. Collagen, as a good example, is often employed as a scaffold for cells since it is the most common protein in the body [18]. Chitosan, the other natural polymer, is an amino polysaccharide derived from chitin. This non-toxic and biocompatible material is easily used to construct matrices with varying degrees of porosity. Therefore, it has a high potential in tissue engineering applications and wound healing [19]. Hence, matrices composed of collagen and chitosan may create an appropriate environment for the regeneration of skin tissue [3]. Nevertheless, both of these materials are hemostatic and their mechanical properties and biodegradation rates are not good [19].
In this study, we have fabricated two matrices using natural collagen/chitosan and synthetic PCL polymers by different manufacture methods, solvent casting and electrospinning, respectively. Then, the electrospun PCL substrate and the collagen/chitosan film were implanted into the same rat models to investigate whether the material substance was more important for wound healing or surface topography of substrates.
PCL (Mw 80,000) (Sigma, New York, NY, USA) was dissolved in N-dimethylformamide and chloroform (Merck, Kenilworth, NJ, USA) by ratio 1/9 (N-dymethylformamid/chloroform). Spinning solution with concentration of 8% (w/v) was prepared. Then, the solution was electrospun upon applying a high voltage (22.5 kv) and mass flow rate of 1 ml/h at room temperature. Polymer nanofibers were collected on an aluminum foil which covered the target [1].
Collagen-chitosan film was developed by casting and solvent-evaporation method. Collagen (type I, Sigma) and chitosan (Sigma) were separately dissolved in acetic acid (0.5 M, Merck). Mixture of the 1% collagen and 1% chitosan solutions (9:1 V/V) were cast on polystyrene molds, frozen at -80℃ for 2 hours and then lyophilized in a freeze dryer for 24 hours. Scaffolds then cross-linked using 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (Sigma). The sample was rinsed in distilled water and dried at 37℃ for 4 days.
The morphology and surface topography of PCL nanofibers and collagen-chitosan film were visualized by scanning electron microscopy (SEM; model LEO 1455 vp, Firma Zeiss, Oberkochen, Germany). The scaffolds were coated with gold using a sputter coater and imaged. Surface properties of substrates were evaluated and Fiber diameters and size distribution of PCL nanofibers were measured from SEM images using Image J software (National Institutes of Health, Bethesda, MD, USA).
Tensile properties of electrospun PCL substrate and collagen-chitosan film were determined by using Wance material testing machine, equipped with a 5 kN load cell. The sample of PCL scaffold with 11 mm width and 40 mm length in ~154 µm thickness and collagen-chitosan film with 11 mm width and 50 mm length in ~128 µm thickness were evaluated. The crosshead speed was set at 10 mm/min and the analyses were performed at ambient conditions. Tensile strength, elastic modulus, and tensile strain were obtained from the stress-strain curves generated by the testing machine.
Ten Wistar rats, weighting about 180 g, was anesthetized by intra peritoneal injection of 20 mg/kg ketamine and 10 mg/kg xylazine. The back area's hairs of the rats were shaved, then sterilized by 70% ethanol. Two full thickness circular excisions (about 20 mm diameter) were made on the back area's skin of the animals.
PCL nanofibers and collagen-chitosan film were sterilized by immerging into 70% ethanol for 1 hour then immerged into phosphate buffered saline (PBS; pH 7.2) to eliminate the ethanol. PCL nanofibers and collagen/chitosan films (about 20 mm diameter) were implanted on the wound sites. After 2 weeks, the implantation sites were excised and their cellular structures and the expression of epidermal protein marker, pancytokeratin, were investigated by histological and immunohistochemical evaluation.
Tissue-engineered skin samples were fixed in neutral buffered formalin (10%) for a week, dehydrated in a series of increasing ethanol concentrations and embedded in paraffin. Sections (5 mm) were stained with hematoxylin and eosin (H&E), silver and Mason's trichrome methods. For immunohistochemistry, sections were deparaffinized and incubated in methanol containing 0.3% H2O2 for 15 minutes at room temperature for blocking of peroxides activity. Antigen retrieval was performed with 10 mm sodium citrate buffer (pH 6) for 20 minutes at 95℃. Sections were incubated with 5% goat serum in PBS at room temperature and then were incubated for 30 minutes at room temperature with mouse monoclonal antibody against pancytokeratin (Santa Cruz Biotechnology, Heidelberg, Germany) diluted 1:100 in blocking solution. After three washes in PBS, the samples were incubated with secondary antibody for 30 minutes at room temperature. Following three washes in PBS, the sections were treated with a 3,3'-diaminobenzidine substrate. Positive immunoreactivity was visualized as brown stain. Suitable positive and negative controls was also set for correct interpretation.
PCL nanofibers seemed to be distinctly separated and randomly distributed. Individual nanofibers were recognized to be continuous and cylindrical. Fiber diameter was estimated ranging from 460 nm to 3.5 µm whereas 75% of fibers were <1,500 nm. In contrast, collagen-chitosan film was almost smooth but few irregularities were observed in some areas (Fig. 1A, B).
Mechanical stiffness of PCL substrate and collagen-chitosan film was assessed in terms of averaged tensile strength, elastic modulus, and tensile strain (Table 1). Table 1 demonstrates that elastic modulus was significantly higher in collagen-chitosan film (~45 fold) than that of PCL scaffold. However, the tensile strain was significantly reduced (~25 fold). Tensile strength of the collagen-chitosan film was obtained ~1.8 fold of PCL scaffold (P<0.05). According to these results, despite superior strength of collagen-chitosan film, its plasticity decreased significantly.
On day 0, two circular full thickness wounds (20 mm diameter) were made in each animals for two groups (Fig. 2A). On day 14, to take photographs of the un-epithelialized wound area, the surrounding hair in some groups were re-shaved to clearly show the wound margin. As it was shown in Fig. 2B, the surface area of un-epithelialized wounds were found to be 25% and 13% of primary wound in the collagen/chitosan film and PCL nanofibers implantation groups, respectively (P<0.05).
H&E staining images revealed that good levels of cellularization and formation of skin-like tissue including epidermis and dermis structures, have accomplished in engrafted wound with PCL matrix after 2 weeks. Keratin layer was also seen that is almost similar to native skin. No inflammation or infection was observed in the harvest skin. In the wound engrafted with collagen-chitosan film, trace of the scaffold could be identified instead of dermis layer and no determinable epithelium could be observed after 2 weeks. In addition, Small vessels invaded to the scaffold and a few inflammatory cells could be observed in the scaffold surrounding the blood vessels (Fig. 3A, B). Silver and trichrome staining for demonstration of thin and coarse bundles of collagen declared that a thin network of collagen fibers (delicate collagen) was formed under the epidermis in the superficial papillary layer of dermis, around the hair follicles and sebaceous glands in the PCL group. Furthermore, thick bundles of collagen fibers were also evident in the deeper reticular layer of dermis, almost similar to the native skin in the same group (Fig. 3C, D, E, F). Taken together, these findings emphasized on the similarities of healed wound by PCL to normal skin.
Immunohistochemical study was also performed to detect expression of pan-cytokeratin in samples sections. This protein was highly expressed in PCL engrafted sample in all cell layers. Despite high level expression of cytokeratin in PCL graft group, the expression of this marker was weak in collagen/chitosan one because of lack of apparently developed epithelium (Fig. 4A, B).
Skin, the largest organ of the body, is composed of two layers: the epidermis, an epithelial layer of ectodermal origin, and the dermis, a layer of mesodermal connective tissue. The present study was designed to investigate the healing potential of two different matrices with different materials substance (PCL and collagen-chitosan), surface topographies and physical properties for skin tissue engineering. The results suggest that PCL substrate has a more ideal property from both physical property and wound healing aspects than collagen-chitosan film. Besides, PCL nanofibers exhibited lower tensile strength and elastic modulus when compared with collagen-chitosan film, indicating that PCL fibers might be more elastic than the collagen-chitosan film and hence suitable for skin tissue engineering [41214].
Many studies have shown that skin fibroblasts and keratinocytes can sense their microenvironmental cues, including the physical properties and surface topography of a substrate [242021]. Substrate topography and mechanical properties have been shown to have a noticeable influence on the cell proliferation, gene expression, and wound healing [821]. Although many studies support this suggestion that the fibrous structure of a scaffold is more efficient in skin tissue engineering [24222324], several studies have supported the wound healing potential of matrices fabricated from collagen and chitosan [25262728]. Furthermore, cells behavior in a 3D scaffold is different from those on flat surfaces [1229]. According to a previous study, cellular responses of both keratinocyte and fibroblast on the fibrous chitosan scaffolds were considerably better than those on the film counterparts due to the larger surface area of the fibrous scaffolds to cell attachment [2]. Kuppan et al. [21] also demonstrated that electrospun poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) fibers are more efficient in cell proliferation, gene expression and wound healing when compared with PHBV 2D films. The aforementioned studies are in favor of our results regarding PCL graft. According to our evidence, fibrous structure of PCL matrix seems to make a construction similar to the extracellular matrix in vivo that speed up migration of neighbor fibroblasts and immature keratinocytes to the wound site and generate dermal and epidermal like structures, respectively. In conclusion, our study suggests that surface topography is a more determining factor than material substance in skin wound healing and nanofibrous matrices, even though fabricated from synthetic polymers, have favorable properties to skin cells migration and penetration as compared with natural polymers' smooth film.
Figures and Tables
Fig. 1
Scanning electron microscopy images of collagen/chitosan film (A) and polycaprolactone nanofibers matrix (B). Scale bars = 20 µm.

Fig. 2
Wounds appearances before implantation on day 0 (A) and after implantation of collagen/chitosan film and polycaprolactone (PCL) nanofiber on day 14 (B).
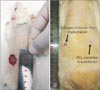
Fig. 3
Hematoxylin and eosin (A, B), silver (C, D), and Mason's trichrome (E, F) histological stainings in different groups on day 14. Note the apparent epidermis and organized dermis composed of papillary and reticular layers in PCL nanofibers implant as compared with collagen/chitosan group. DC, delicate collagen; Epi, epidermis; HF, hair follicle; K, keratinized layer; PCL, polycaprolactone; PL, papillary layer of dermis; SG, sebaceous glands; TBC, thick bundle of collagen. Scale bars=25 µm (A-D).
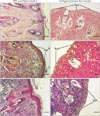
Fig. 4
Immunological staining of epidermal marker protein (pan-cytokeratin) in different groups on day 14. Epithelialization, skin appendages with epidermal origin, i.e., hair follicle (HF) and sebaceous gland, were clearly evident in polycaprolactone nanofibers implant (A) as compared with collagen/chitosan implant (B). Epi, epidermis; BL, basal layer. Scale bars=25 µm (A, B).

Table 1
Basic characteristics of polycaprolactone (PCL) nanofibers and collagen/chitosan film

Acknowledgements
This work was financially supported by Ahvaz Jundishapur University of Medical Sciences and conducted at cellular and molecular research center (CMRC).
References
1. Hejazian LB, Esmaeilzade B, Moghanni Ghoroghi F, Moradi F, Hejazian MB, Aslani A, Bakhtiari M, Soleimani M, Nobakht M. The role of biodegradable engineered nanofiber scaffolds seeded with hair follicle stem cells for tissue engineering. Iran Biomed J. 2012; 16:193–201.
2. Neamnark A, Sanchavanakit N, Pavasant P, Rujiravanit R, Supaphol P. In vitro biocompatibility of electrospun hexanoyl chitosan fibrous scaffolds towards human keratinocytes and fibroblasts. Eur Polym J. 2008; 44:2060–2067.
3. Ma L, Gao C, Mao Z, Zhou J, Shen J, Hu X, Han C. Collagen/chitosan porous scaffolds with improved biostability for skin tissue engineering. Biomaterials. 2003; 24:4833–4841.
4. Jayarama Reddy V, Radhakrishnan S, Ravichandran R, Mukherjee S, Balamurugan R, Sundarrajan S, Ramakrishna S. Nanofibrous structured biomimetic strategies for skin tissue regeneration. Wound Repair Regen. 2013; 21:1–16.
5. Dhandayuthapani B, Yoshida Y, Maekawa T, Kumar DS. Polymeric scaffolds in tissue engineering application: a review. Int J Polym Sci. 2011; 2011:290602.
6. Craighead HG, Turner SW, Davis RC, James C, Perez AM, St. John PM, Isaacson MS, Kam L, Shain W, Turner JN, Banker G. Chemical and topographical surface modification for control of central nervous system cell adhesion. Biomed Microdevices. 1998; 1:49–64.
7. Hsu SH, Chen CY, Lu PS, Lai CS, Chen CJ. Oriented Schwann cell growth on microgrooved surfaces. Biotechnol Bioeng. 2005; 92:579–588.
8. Cao H, McHugh K, Chew SY, Anderson JM. The topographical effect of electrospun nanofibrous scaffolds on the in vivo and in vitro foreign body reaction. J Biomed Mater Res A. 2010; 93:1151–1159.
9. Berry CC, Campbell G, Spadiccino A, Robertson M, Curtis AS. The influence of microscale topography on fibroblast attachment and motility. Biomaterials. 2004; 25:5781–5788.
10. Sangsanoh P, Waleetorncheepsawat S, Suwantong O, Wutticharoenmongkol P, Weeranantanapan O, Chuenjitbuntaworn B, Cheepsunthorn P, Pavasant P, Supaphol P. In vitro biocompatibility of schwann cells on surfaces of biocompatible polymeric electrospun fibrous and solution-cast film scaffolds. Biomacromolecules. 2007; 8:1587–1594.
11. Conconi MT, Lora S, Baiguera S, Boscolo E, Folin M, Scienza R, Rebuffat P, Parnigotto PP, Nussdorfer GG. In vitro culture of rat neuromicrovascular endothelial cells on polymeric scaffolds. J Biomed Mater Res A. 2004; 71:669–674.
12. Ng R, Zang R, Yang KK, Liu N, Yang ST. Three-dimensional fibrous scaffolds with microstructures and nanotextures for tissue engineering. RSC Adv. 2012; 2:10110–10124.
13. Grayson WL, Ma T, Bunnell B. Human mesenchymal stem cells tissue development in 3D PET matrices. Biotechnol Prog. 2004; 20:905–912.
14. Izquierdo R, Garcia-Giralt N, Rodriguez MT, Cáceres E, García SJ, Gómez Ribelles JL, Monleón M, Monllau JC, Suay J. Biodegradable PCL scaffolds with an interconnected spherical pore network for tissue engineering. J Biomed Mater Res A. 2008; 85:25–35.
15. Woodruff MA, Hutmacher DW. The return of a forgotten polymer: polycaprolactone in the 21st century. Prog Polym Sci. 2010; 35:1217–1256.
16. Yoshimoto H, Shin YM, Terai H, Vacanti JP. A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomaterials. 2003; 24:2077–2082.
17. Almany L, Seliktar D. Biosynthetic hydrogel scaffolds made from fibrinogen and polyethylene glycol for 3D cell cultures. Biomaterials. 2005; 26:2467–2477.
18. Pham QP, Sharma U, Mikos AG. Electrospinning of polymeric nanofibers for tissue engineering applications: a review. Tissue Eng. 2006; 12:1197–1211.
19. Wang XH, Li DP, Wang WJ, Feng QL, Cui FZ, Xu YX, Song XH, van der Werf M. Crosslinked collagen/chitosan matrix for artificial livers. Biomaterials. 2003; 24:3213–3220.
20. Han CM, Zhang LP, Sun JZ, Shi HF, Zhou J, Gao CY. Application of collagen-chitosan/fibrin glue asymmetric scaffolds in skin tissue engineering. J Zhejiang Univ Sci B. 2010; 11:524–530.
21. Kuppan P, Vasanthan KS, Sundaramurthi D, Krishnan UM, Sethuraman S. Development of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) fibers for skin tissue engineering: effects of topography, mechanical, and chemical stimuli. Biomacromolecules. 2011; 12:3156–3165.
22. Kobsa S, Kristofik NJ, Sawyer AJ, Bothwell AL, Kyriakides TR, Saltzman WM. An electrospun scaffold integrating nucleic acid delivery for treatment of full-thickness wounds. Biomaterials. 2013; 34:3891–3901.
23. Gandhimathi C, Venugopal JR, Bhaarathy V, Ramakrishna S, Kumar SD. Biocomposite nanofibrous strategies for the controlled release of biomolecules for skin tissue regeneration. Int J Nanomedicine. 2014; 9:4709–4722.
24. Jin G, Prabhakaran MP, Kai D, Annamalai SK, Arunachalam KD, Ramakrishna S. Tissue engineered plant extracts as nanofibrous wound dressing. Biomaterials. 2013; 34:724–734.
25. Nunes PS, Albuquerque RL Jr, Cavalcante DR, Dantas MD, Cardoso JC, Bezerra MS, Souza JC, Serafini MR, Quitans LJ Jr, Bonjardim LR, Araújo AA. Collagen-based films containing liposome-loaded usnic acid as dressing for dermal burn healing. J Biomed Biotechnol. 2011; 2011:761593.
26. Ramasamy P, Shanmugam A. Characterization and wound healing property of collagen-chitosan film from Sepia kobiensis (Hoyle, 1885). Int J Biol Macromol. 2015; 74:93–102.
27. Li W, Guo R, Lan Y, Zhang Y, Xue W, Zhang Y. Preparation and properties of cellulose nanocrystals reinforced collagen composite films. J Biomed Mater Res A. 2014; 102:1131–1139.
28. Li X, Nan K, Li L, Zhang Z, Chen H. In vivo evaluation of curcumin nanoformulation loaded methoxy poly(ethylene glycol)-graft-chitosan composite film for wound healing application. Carbohydr Polym. 2012; 88:84–90.
29. Li Y, Yang ST. Effects of three-dimensional scaffolds on cell organization and tissue development. Biotechnol Bioprocess Eng. 2001; 6:311–325.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download