Abstract
Mast cells are known as effector cells of IgE-mediated allergic responses, but role of mast cells in contact hypersensitivity (CHS) has been considered controversial. In this study, we investigated role of mast cell in trimellitic anhydride (TMA)-induced CHS. The mice were sensitized to TMA on the back and repeatedly challenged with TMA on the left ear at 1-week intervals. The ear after challenge showed biphasic responses. The repetition of TMA challenge shifted in time course of ear response and enlarged the extent of early and late phase reactions in proportion to the frequency of TMA challenges in C57BL/6 mice. In late phase reaction, peak of ear response by single challenge showed at 24 hours after challenge, but the peak by repeat challenges at 8 hours after the last challenge. Number of mast cells and eosinophils per unit area increased in proportion to frequency of TMA challenges. However, mast cell-deficient WBB6F1/J-KitW/KitW-v mice developed the late phase reaction without the early phase reaction. The repetition of TMA challenge shifted in time course of ear response and enlarged the extent of ear response and the infiltration of eosinophils. The magnitude of these responses observed according to the frequency of the TMA challenge in mast cell-deficient WBB6F1/J-KitW/KitW-v mice was significantly lower than that in C57BL/6 mice. Also TMA elicited mast cell degranulation and histamine release from rat peritoneal mast cells in a concentration-dependent manner. Conclusively, TMA induces the early and late phase reactions in CHS, and mast cells may be required for TMA-induced CHS.
Contact hypersensitivity (CHS) is a frequently observed inflammatory disease of skin with a high socioeconomic relevance [1]. The origin and nature of the compounds have a potential to induce a contact sensitivity are very diverse, but they share some common features: contact allergens are small molecular weight chemical named haptens, that are not immunogenic by themselves and need to bind to epidermal proteins. And they act as carrier proteins to form the hapten-carrier complex that finally act as the antigen [2].
The process of CHS induced by these haptens occurs in two distinct phases. In the first phase, the hapten penetrates into the viable layers of the epidermis and into the various cells at contact site. At these sites, the hapten must react with carrier protein or peptide to form a complete antigen. Skin resident Langerhans cells take up the modified proteins and prime allergen-specific T cell in the skin-draining lymph node, a process called "induction or sensitization." In second phase, known as "elicitation," re-exposure to the inducing hapten reactivates these memory T cells to produce a variety of proinflammatory cytokines that trigger the inflammatory response [3].
Following challenge with relevant haptens, sensitized animals exhibit an acute biphasic skin reaction. An early phase reaction peaked 1 hour after hapten challenge, and seems to be mediated by mast cell degranulation. Since receptor antagonists for histamine, the major mediator of mast cells, reduce the reaction [4]. A late phase reaction appeared 6-30 hours after the hapten challenge. This local CHS reaction was characterized by marked infiltrations of inflammatory cells such as eosinophils, lymphocytes and neutrophils [5]. However, the mechanism of biphasic reaction is not completely understood.
Trimellitic anhydride (TMA), a known respiratory sensitizer that induced occupational asthma [6], is widely used to trigger T-cell-dependent CHS reaction in mice and elicits eosinophil and T-cell infiltration, Th2 cytokine production, and IgE increase. In the TMA-induced CHS models, mice are sensitized on flank skin and T-cell-dependent skin inflammation is induced by topical challenges of ears. The TMA-induced cutaneous inflammation showed increase of cytokine profile, infiltration of immune cells, and increase of serum IgE levels [78910].
It has been reported that mast cells are well known as effector cells of IgE-mediated allergic responses, but over the past years important functions of mast cells in innate and adaptive immunity, pathogen defense, and autoimmune diseases [11121314]. In the skin, the number of mast cells was proportional to the ability of 2,4,6-trinitro-1-chrolobenzene (TNCB)-treated BALB/c and mast cell-deficient WBB6F1/J-KitW/KitW-v mice to mount an immediate type response to TNCB suggested that mast cell accumulating at the site of antigen application were a prerequisite for the development of an immediate type response to contact sensitizing agents [15]. Also, Togawa et al. [5] demonstrated the role of interleukin (IL)-4, IL-5 and mast cells in the accumulation of eosinophils during allergic cutaneous late phase reaction in mice. They showed that IL-4, IL-5 and mast cells play an important role in IgE and CD4+ T cell-mediated cutaneous late phase, but differently regulate the response. IL-5 may play an important role in modulating the eosinophil recruitment, while IL-4 contributes to the development of edema in cutaneous late phase response in mice. And IgE-mediated mast cell activation was required for full response [5]. However, others described undiminished CHS under conditions of mast cell deficiency [1617]. Recently, Grimbaldeston et al. [18] reported mast cells suppressed contact dermatitis.
Although many reports suggested a regulatory role for mast cells in CHS by contact sensitizing agent, but the exact role of the mast cells is still controversial. So, this study was to investigate whether the role of mast cells in early and late phase reactions of the TMA-induced CHS.
C57BL/6 male mice aged 6 weeks and Sprague-Dawley rat weighing 250-300 g purchased from Korean Damool Science (Daejeon, Korea). Mast cell-deficient WBB6F1/J-KitW/KitW-v (W/Wv) mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). Each experimental group consisted of 5 mice. All experiments were repeated at least two times with similar results. They were housed the experiments in a laminar flow cabinet and article lighting conditions with 12 hours day/night cycle and had access to food and water ad libitum.
TMA was purchased from Sigma (St. Louis, MO, USA). Acetone (Junsei, Tokyo, Japan):olive oil (Filippo Berio, Lucca, Italy) (4:1) was used as vehicle.
Mice were sensitized on shaved back skin with 100 µl of 50 mg/ml TMA in a 4:1 acetone:olive oil solution (A/O) on day 0 and 50 µl of 12.5 mg/ml TMA in A/O on day 7 under light anesthesia according modified method of Chai et al. [10]. On days 14, 21, 28, and/or 35, each left ear was repeatedly challenged with 20 µl of 2 mg/ml TMA in A/O and each right ear was repeatedly with 20 µl of A/O (Fig. 1).
The ear thickness just before and after the last challenge was measured three times with a dial thickness gauge (Model 7326, Mitutoyo Manufacturing, Tokyo, Japan), and the difference was defined as ear swelling and expressed in units of 10-4 inches (mean±SEM). In the time-course study, the ear thickness was measured at 1, 2, 4, 8, 12, 24, 48, and 72 hours after challenge.
Seventy-two hours after the last challenge, animals were sacrificed and both ears of animals were excised. Specimens were fixed immediately by immersion in 10% neutral buffered formalin solution for 12 hours at 4℃. The fixed specimens were dehydrated in a graded series of alcohols, embedded in paraffin. The sections were cut at 4 µm by microtome (SM 2000R, Leica, Jena, Germany) and stained with Congo red solution [19]. In the congo red method, the slides were stained in hematoxylin solution for 3 seconds and rinsed in alkaline sodium chloride solution for 20 minutes. The slides were stained in congo red solution for 2 hours, washed in distilled water, dehydrated in graded ethanol and cleared in xylene, the cover slipped.
Seventy-two hours after the last challenge, animals were sacrificed and both ears of animals were excised. Specimens were fixed immediately by immersion in Carnoy's solution for 12 hours at 4℃. The fixed specimens were dehydrated in a graded series of alcohols, embedded in paraffin. The sections were cut at 4 µm by microtome (SM 2000R, Leica) and stained with alcian blue solution. In the alcian blue staining method, the sections were stained with 1% (W/V) alcian blue (pH 1.0) in 0.7 N HCl for 1 hour at 37℃, washed running tap water for 5 minutes and countstained for 10 minutes and dried in air.
Rat peritoneal mast cells (RPMCs) were isolated as previously described [20]. Briefly, rats were anesthetized by ether and injected with 10 ml of HEPES-Tyrode buffer into the peritoneal cavity, and the abdomen was gently massaged for about 90 seconds. The peritoneal cavity was opened, and the fluid was aspirated and RPMC were purified by using a percoll density gradient. The purify and viability of cells were tested by toluidine blue staining and trypan blue exclusion (both >95%), respectively. Purified mast cells were resuspended in HEPES-Tyrode buffer. Mast cells were observed under phase contrast and photographed.
Purified mast cell suspensions were preincubated to 37℃ in HEPES-Tyrode buffer for 10 minutes and then incubated with the various concentration of TMA for 30 minutes. The reaction was stopped by centrifugation at 150 ×g for 10 minutes and histamine content in the supernatant was measured by the radioenzymatic method of Chai et al. [20]. Histamine release was calculated in percent of the total content of the cell suspension and corrected for spontaneous release occurring in the absence of TMA. Total histamine content was determined by 10 minutes boiling of aliquots of mast cells from the same animals in each experiment.
A CHS was investigated during repeated challenge for testing CHS to TMA in C57BL/6 mice. The sensitized mice were challenged with 100 mg/ml TMA to the left ear on days 14, 21, and 35. The changes of ear thickness on time course following 100 mg/ml TMA challenge or vehicle application on the ear of C57BL/6 mice are shown in Fig. 2. Single challenge of TMA induced biphasic ear swelling response which consisted of an early phase reaction and a late phase reaction. The early phase reaction peaked at 1 hour and the late phase reaction at 24 hours after challenge, respectively. The repetition of the TMA challenge shifted in the time course of ear swelling response and enlarged the extent of early and late phase reactions in proportion to the frequency of TMA challenge in C57BL/6 mice. The extent of the late phase reaction induced by the repeated challenge with TMA was dependent on the extent of the early phase reaction. The larger of the extent of early phase reaction's increase, the bigger of the extent of late phase reaction's increase. In the late phase reaction, the peak of ear swelling response by single challenge showed at 24 hours after challenge, but peak by the repeat challenges at 8 hours after challenge. However no shift of the reaction peak to the early phase reaction was observed. There were significant differences in the ear swelling response in vehicle versus TMA-challenged mice and single TMA-challenged versus repeatedly TMA-challenged mice. The extent of an early and late phase reactions increased once< or ≤twice <<four times of TMA challenge.
The infiltrations of eosinophils and mast cells were measured as a cellular mechanism underlying the ear swelling response. Histologic examinations were observed at 72 hours after the TMA challenge or vehicle application on the ear of C57BL/6 mice which sensitized with TMA on the back skin at day 0 and 7. As shown Figs. 3 and 4, TMA challenge induced the significant infiltrations of eosinophils and mast cells into the dermis of the ear in C57BL/6 mice. In addition, the extent of eosinophilia depended on the number of TMA challenge times. Dilatation of blood vessels in the dermis of skin was observed. Also, plugs of eosinophils in the ear of mice over twice TMA challenges were observed in stratum corneum of the epidermis. Table 1 showed that the number of eosinophils and mast cells per unit area in the ear of C57BL/6 mice increased in proportion to the frequency of TMA challenges. There were significant differences in the infiltration of eosinophils and mast cells in vehicle versus TMA-challenged mice and single TMA-challenged versus repeatedly TMA-challenged mice. The number of eosinophils and dermal mast cells per unit area (mm2) increased once< or ≤twice <<four times of TMA challenge.
Ear swelling responses in mast cell-deficient W/WV mice were measured in order to investigate the roles of mast cells in the CHS by repeated TMA challenge in mice. The sensitized mice were challenged with 100 mg/ml TMA to the left ear on days 14, 21, 28, and 35. The changes of ear thickness on time course following 100 mg/ml TMA challenge or vehicle application on the ear of mast cell-deficient W/Wv mice are shown in Fig. 5. Mast cell-deficient W/Wv mice showed the only late phase reaction, while congenic normal mice showed both the early and the late phase reactions. The repetition of the TMA challenge shifted in the time course of ear swelling response and enlarged the extent of late phase reaction in proportion to the frequency of TMA challenges in mast cell-deficient W/Wv mice. The late phase reaction peaked at 24 hours after single challenge, but peak by the repeat challenges at 8 hours after the challenges. These results suggest that mast cells may be involved in CHS by repeatedly TMA challenge. The magnitude of these responses observed according to the frequency of the TMA challenge was significantly lower than that in normal mice. These results strongly suggest that the increase of early phase reaction by repeatedly TMA challenge and the extent of late phase reaction's increase may dependent on the mast cells at the site of inflammation.
The infiltrations of eosinophils and mast cells were measured as a cellular mechanism underlying the ear swelling response. Histologic examinations were observed at 72 hours after the TMA challenge or vehicle application on the ear of mast cell-deficient W/Wv mice which sensitized with TMA on the back skin at day 0 and 7. As shown Fig. 6, TMA challenge induced the significant infiltrations of eosinophils into the dermis of the ear in mast cell-deficient W/Wv mice. In addition, the extent of eosinophilia depended on the number of TMA challenge times. Also, plugs of eosinophils in the ear of mice over twice TMA challenges were observed in stratum corneum of the epidermis. But the extent of eosinophilia observed according to the frequency of the TMA challenge was significantly lower than that in normal mice. Mast cells weren't found in the dermis of ear in mast cell-deficient W/Wv mice. Table 2 showed the number of eosinophils and mast cells per unit area in the ear of mast cell-deficient W/Wv mice. There were significant differences in the infiltration of eosinophils in C57BL/6 verus mast cell-deficient W/Wv mice.
Direct effects of TMA on mast cell activation in vivo were investigated in order to investigate the roles of mast cells in the CHS by repeated TMA challenge in mice. TMA induced the mast cell degranulation and histamine release from RPMCs (Table 3, Fig. 7). The mast cell in HEPES-Tyrode buffered solution showed clear, smooth outline, spheroidal shape and contained many retractile granules in cytoplasm (Fig. 7A). After the stimulation with 10 mg/ml TMA, the cell became swollen and had many vacuoles, and extruded granules near cell surface and in the surrounding medium (Fig. 7B). TMA induced the mast cell degranulation and the histamine release from rat peritoneal meast cells in a concentration-dependent manner (Table 3).
In 1935, Landsteiner and Jacobs [21] showed that epicutaneous application of small reactive compounds resulted in the induction of CHS. The first treatment (sensitization) had no visible effect, but when the same hapten was applied after a week for a second time (elicitation), a local inflammation at the site of application occurred with a delay of 24-48 hours. The inflammatory reaction is caused by cytokines that are released by antigen (hapten)-specific, sensitized T cells [22]. Histological examination revealed the infiltration of basophils and eosinophils in the superficial dermis as the characteristics of the animal skin reaction [23]. In morphologic study of allergic contact dermatitis and delayed CHS in man skin reactions [1024], a number of important features were found to characterize these reactions, including infiltration and piecemeal degranulation of basophils; degranulation and replication of fixed tissue mast cells; infiltration of eosinophil and neutrophils; increased vascular permeability leading to dermal and epidermal edema, vascular compaction, and erythrocyte extravasation; microvascular alterations affecting endothelial cells and pericytes, with compromise of vessel lumina and basement membrane thickening.
In these studies, repeated application of TMA induced biphasic ear swelling responses which consisted of an early and a late phase reactions in C57BL/6 mice. At the repeatedly challenged skin sites, the dermal mast cell count was about two times higher than the count in normal mice. And, TMA challenge induced the significant infiltrations of eosinophils into the dermis of the ear in C57BL/6 mice. In addition, the extent of eosinophilia depended on the number of TMA challenge times. Also, plugs of eosinophils in the ear of mice over two times of TMA challenge were observed in stratum corneum of the epidermis. The extent of an early and late phase reactions and the number of eosinophils and dermal mast cells per unit area (mm2) in a murine model of CHS increased once< or ≤twice <<four times of TMA challenge.
Some reports previously have shown that antigenic or hapten challenge of mice and rat producing IgE antibody [6252627] causes biphasic ear swelling peaking 1 hour and 24 hours [28]. The ear swelling at 24 hours was accompanied by a marked eosinophil accumulation at the site of inflammation. It is reasonable to assume that antigen introduced into ear skin activates CD4 T cells as well as mast cells at the site of inflammation. Interestingly, CD4 T cells, particularly Th2 cells, and mast cells have been shown to produce common cytokines, so-called Th2 cytokines including IL-4 and IL-5, in vitro [29] and in vivo [30]. These cytokines have been reported to be involved in the eosinophil recruitment, suggesting that either CD4 T cells, mast cells, or both, play a role in the development of antigen-induced CHS. It has been reported recently that injection of chymase, a chymotrypsin-like serine protease stored within mast cell granules, not only increased vascular permeability but also induced leukocytes accumulation in vivo [3132]. Intradermal injection of human chymase into the mouse ear elicited an edematous skin reaction in a biphasic manner, followed by a delayed response persisting for at least 24 hours and induced accumulation of inflammatory cells in the later phases of the skin reaction. A chymase inhibitor and histamine receptor antagonist inhibited the chymase-induced skin reaction. In addition, human chymase showed chemotactic activity to human polymorphonuclear leukocytes in vitro [33]. These findings strongly suggest that mast cell chymase may participate in the two phases of allergic skin inflammation by two distinct mechanisms, i.e., histamine- and leukocyte-dependent mechanisms, respectively [33]. Natsuaki et al. [34] reported the fact that no immediate reaction occurred in the ear without an increase in mast cells suggests that not only IgE antibodies in the blood but also a local increase in mast cells is important for an immediate response to occur. In BALB/c mice, an immediate reaction occurred after the fifth challenge, whereas a sixth challenge after an interval of 3 or 6 months caused only a late phase reaction and no early phase reaction. These animals had an anti-DNP IgE antibody titer comparable to that in mice with early phase reaction, but the dermal mast cell of the left ear was decreased to 2/3 of that in mice with early phase reaction. Togawa et al. [5] showed that IL-4, IL-5, and mast cells play an important role in IgE and CD4+ T cell-mediated cutaneous late phase, but differently regulate the response. IL-5 may play an important role in modulating the eosinophil recruitment, while IL-4 contributes to the development of edema in cutaneous late phase response in mice. And IgE-mediated mast cell activation was required for full response [5]. It was still unknown to what extent dermal mast cells are required for the development of a detectable immediate reaction.
In this study, repeatedly TMA induced the only late phase reaction in mast cell-deficient W/Wv mice, while congenic normal mice showed both the early and the late phase reactions. The repetition of the TMA challenge shifted in the time course of ear swelling response and enlarged the extent of the late phase reaction in proportion to the frequency of TMA challenges in mast cell-deficient W/Wv mice, but less than those in normal mice. The late phase reaction peaked at 24 hours after single challenge, but peak by the repeat challenges at 8 hours after the last challenges. Also TMA elicited mast cell degranulation and histamine release from RPMCs in a concentration-dependent manner in vitro.
Some reports have shown that TMA elicited dose-dependent production of specific IgE and IgG after single and repeated topical skin exposure and an increase in total serum IgE, active sensitization of tissue mast cells, and secretion of IL-4 protein and induction of IL-5 mRNA expression by draining lymph node cells [6273536].
Kitagaki et al. [15] showed that frequent application of a hapten causes the peak skin reaction to shift from a delayed to an immediate one, and they showed that the local cytokine profile at the site of frequent application is shifted Th1 to Th2 [37]. Differences in the properties and the application frequency of haptens, and in animal strain may help to explain these differences in the reaction, such as patterns of IgE response and cytokine expression.
In our experiments, TMA challenges of ears of C57BL/6 mice caused a biphasic ear swelling peaking at 1 hour (early reaction) and 24 hours (late reaction). However, mast cell-deficient WBB6F1/J-KitW/KitW-v mice developed late phase reaction without the early phase reaction which was significantly lower than in C57BL/6 mice.
Conclusively, TMA induces the early and late phase reactions in CHS, and mast cells may be required for TMA-induced CHS.
Figures and Tables
Fig. 1
Schematic diagram of the experimental protocol. Mice were sensitized on shaved back skin with 100 µl of 50 mg/ml tissue microarray (TMA) in a 4:1 acetone: olive oil solution (A/O) on day 0 and 50 µl of 12.5 mg/ml TMA in A/O on day 7. On days 14, 21, 28, and 35, each left ear was repeatedly challenged with 20 µl of 2 mg/ml TMA in A/O and each right ear was repeatedly with 20 µl of A/O.

Fig. 2
(A, B) Ear swelling response on time course following vehicle or tissue microarray (TMA) challenges on the ear of the C57BL/6 mice sensitized on the back skin with TMA. Significant responses were recognized in the vehicle-applied ears compared with the TMA-challenged ears (*P<0.05, ***P<0.001, ###P<0.001).

Fig. 3
Light micrographs of infiltration of eosinophils on 72 hours after vehicle (A) or one time (B) and four times (C) of the tissue microarray (TMA) challenges on the ear of the C57BL/6 mice sensitized on the back skin with TMA. Congo red stain. Scale bars=10 µm (A-C).

Fig. 4
Light micrographs of infiltration of mast cells on 72 hours after vehicle (A) or one time (B), and four times (C) of the tissue microarray (TMA) challenges on the ear of the C57BL/6 mice sensitized on the back skin with TMA. Alcian blue stain. Scale bars=10 µm (A-C).

Fig. 5
Ear swelling response on time course following vehicle or one time (A), two times (B), and four times (C) of tissue microarray (TMA) challenge on the ear of the mast cell-deficient WBB6F1/J-KitW/KitW-v (open circle) and normal (closed circle) mice sensitized on the back skin with TMA. Significant responses were recognized in the TMA-challend ears of mast cell-deficient WBB6F1/J-KitW/KitW-v compared with those of normal mice (**P<0.01, ***P<0.001).

Fig. 6
Light micrographs of infiltration of eosinophils on 72 hours after vehicle (A) and four times (B) of the tissue microarray (TMA) challenges on the ear of the mast cell-deficient WBB6F1/J-KitW/KitW-v mice sensitized on the back skin with TMA. Congo red stain. Scale bars=10 µm (A, B).
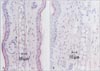
Fig. 7
Light micrographs of rat peritoneal mast cells with HEPES-Tyrode buffered solution (A) or tissue microarray (TMA) (B). The mast cell in HEPES-Tyrode buffered solution showed clear, smooth outline, spheroidal shape and contained many retractile granules in cytoplasm. After the stimulation with 10 mg/ml TMA, the cell became swollen and had many vacuoles, and extruded granules near cell surface and in the surrounding medium. Scale bars=10 µm (A, B).
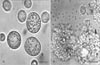
Table 1
The number of eosinophils and mast cells (MCs) per unit area (mm2) on 72 hours after vehicle, or tissue microarray (TMA) repeatedly challenge in the ear of C57BL/6 mice

| Frequency of TMA challenge | No. of eosinophilsa) | No. of MCsb) |
|---|---|---|
| 0 | 3.83±0.31 | 69.23±3.74 |
| 1 | 242.30±12.25** | 108.40±1.24* |
| 2 | 263.30±22.91 | 119.70±3.42 |
| 4 | 573.50±33.82## | 134.60±2.65# |
*P<0.05, **P<0.01: vehicle challenge vs. once TMA challenge. #P<0.05, ##P<0.01: twice TMA challenge vs. four times of TMA challenge. a)The slides were cut at 4 µm-thickness paraffin sections by microtome and stained with Congo red solution. b)The sections were cut at 4-µm-thickness paraffin sections by microtome and stained with alcian blue solution.
Table 2
The number of eosinophils and mast cells (MCs) per unit area (mm2) on 72 hours after vehicle, or tissue microarray (TMA) repeatedly challenge in the ear of mast cell-deficient WBB6F1/J-KitW/KitW-v mice

| Frequency of TMA challenge | No. of eosinophilsa) | No. of MCsb) |
|---|---|---|
| 0 | 2.9±0.31 | 0.0±0.0 |
| 4 | 105.5±38.5* | 0.0±0.0 |
Table 3
Tissue microarray (TMA)-induced mast cell degranulation and histamine release from rat peritoneal mast cells in a concentration-dependent manner

Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2012R1A1A3013857, to Ok Hee Chai; 2012-0025300, to Chang Ho Song) and by research grants from the Korea Food Research Institute.
References
1. Uter W, Schnuch A, Geier J, Frosch PJ. Epidemiology of contact dermatitis: the information network of departments of dermatology (IVDK) in Germany. Eur J Dermatol. 1998; 8:36–40.
2. Saint-Mezard P, Rosieres A, Krasteva M, Berard F, Dubois B, Kaiserlian D, Nicolas JF. Allergic contact dermatitis. Eur J Dermatol. 2004; 14:284–295.
3. Ryan CA, Hulette BC, Gerberick GF. Approaches for the development of cell-based in vitro methods for contact sensitization. Toxicol In Vitro. 2001; 15:43–55.
4. Inagaki N, Sakurai T, Abe T, Musoh K, Kawasaki H, Tsunematsu M, Nagai H. Characterization of antihistamines using biphasic cutaneous reaction in BALB/c mice. Life Sci. 1998; 63:PL145–PL150.
5. Togawa M, Kiniwa M, Nagai H. The roles of IL-4, IL-5 and mast cells in the accumulation of eosinophils during allergic cutaneous late phase reaction in mice. Life Sci. 2001; 69:699–705.
6. Zhang XD, Murray DK, Lewis DM, Siegel PD. Dose-response and time course of specific IgE and IgG after single and repeated topical skin exposure to dry trimellitic anhydride powder in a Brown Norway rat model. Allergy. 2002; 57:620–626.
7. Dearman RJ, Warbrick EV, Skinner R, Kimber I. Cytokine fingerprinting of chemical allergens: species comparisons and statistical analyses. Food Chem Toxicol. 2002; 40:1881–1892.
8. Sailstad DM, Ward MD, Boykin EH, Selgrade MK. A murine model for low molecular weight chemicals: differentiation of respiratory sensitizers (TMA) from contact sensitizers (DNFB). Toxicology. 2003; 194:147–161.
9. Schneider C, Döcke WD, Zollner TM, Röse L. Chronic mouse model of TMA-induced contact hypersensitivity. J Invest Dermatol. 2009; 129:899–907.
10. Chai OH, Lee HK, Lee YC, Lee MS, Han EH, Kim HT, Song CH. Roles of TNF-alpha and IgE in the late phase of contact hypersensitivity induced by trimellitic anhydride. Exp Mol Med. 2005; 37:408–417.
11. Abraham SN, St John AL. Mast cell-orchestrated immunity to pathogens. Nat Rev Immunol. 2010; 10:440–452.
12. Dawicki W, Marshall JS. New and emerging roles for mast cells in host defence. Curr Opin Immunol. 2007; 19:31–38.
13. Grimbaldeston MA, Chen CC, Piliponsky AM, Tsai M, Tam SY, Galli SJ. Mast cell-deficient W-sash c-kit mutant Kit W-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am J Pathol. 2005; 167:835–848.
14. McNeil HP, Adachi R, Stevens RL. Mast cell-restricted tryptases: structure and function in inflammation and pathogen defense. J Biol Chem. 2007; 282:20785–20789.
15. Kitagaki H, Fujisawa S, Watanabe K, Hayakawa K, Shiohara T. Immediate-type hypersensitivity response followed by a late reaction is induced by repeated epicutaneous application of contact sensitizing agents in mice. J Invest Dermatol. 1995; 105:749–755.
16. Galli SJ, Hammel I. Unequivocal delayed hypersensitivity in mast cell-deficient and beige mice. Science. 1984; 226:710–713.
17. Mekori YA, Galli SJ. Undiminished immunologic tolerance to contact sensitivity in mast cell-deficient W/Wv and Sl/Sld mice. J Immunol. 1985; 135:879–885.
18. Grimbaldeston MA, Nakae S, Kalesnikoff J, Tsai M, Galli SJ. Mast cell-derived interleukin 10 limits skin pathology in contact dermatitis and chronic irradiation with ultraviolet B. Nat Immunol. 2007; 8:1095–1104.
19. Song CH, Galli SJ, Lantz CS, Hu X, Stevens RL, Friend DS. 13 Congo red staining of intraepithelial mucosal mast cells. J Histochem Cytochem. 1999; 47:1645C–11645.
20. Chai OH, Shon DH, Han EH, Kim HT, Song CH. Effects of Anemarrhena asphodeloides on IgE-mediated passive cutaneous anaphylaxis, compound 48/80-induced systemic anaphylaxis and mast cell activation. Exp Toxicol Pathol. 2013; 65:419–426.
21. Landsteiner K, Jacobs J. Studies on the sensitization of animals with simple chemical compounds. J Exp Med. 1935; 61:643–656.
22. Homey B, Steinhoff M, Ruzicka T, Leung DY. Cytokines and chemokines orchestrate atopic skin inflammation. J Allergy Clin Immunol. 2006; 118:178–189.
23. Sugawara Y, Okamoto Y, Sawahata T, Tanaka K. Skin reactivity in guinea pigs sensitized with 2,4-toluene diisocyanate. Int Arch Allergy Immunol. 1993; 100:190–196.
24. Dvorak HF, Mihm MC Jr, Dvorak AM. Morphology of delayed-type hypersensitivity reactions in man. J Invest Dermatol. 1976; 67:391–401.
25. Arts JH, Dröge SC, Bloksma N, Kuper CF. Local lymph node activation in rats after dermal application of the sensitizers 2,4-dinitrochlorobenzene and trimellitic anhydride. Food Chem Toxicol. 1996; 34:55–62.
26. Ban M, Hettich D. Relationship between IgE positive cell numbers and serum total IgE levels in mice treated with trimellitic anhydride and dinitrochlorobenzene. Toxicol Lett. 2001; 118:129–137.
27. Vento KL, Dearman RJ, Kimber I, Basketter DA, Coleman JW. Selectivity of IgE responses, mast cell sensitization, and cytokine expression in the immune response of Brown Norway rats to chemical allergens. Cell Immunol. 1996; 172:246–253.
28. Sawada K, Nagai H, Basaki Y, Yamaya H, Ikizawa K, Watanabe M, Kojima M, Matsuura N, Kiniwa M. The expression of murine cutaneous late phase reaction requires both IgE antibodies and CD4 T cells. Clin Exp Allergy. 1997; 27:225–231.
29. Metz M, Siebenhaar F, Maurer M. Mast cell functions in the innate skin immune system. Immunobiology. 2008; 213:251–260.
30. Seder RA, Paul WE, Ben-Sasson SZ, LeGros GS, Kagey-Sobotka A, Finkelman FD, Pierce JH, Plaut M. Production of interleukin-4 and other cytokines following stimulation of mast cell lines and in vivo mast cells/basophils. Int Arch Allergy Appl Immunol. 1991; 94:137–140.
31. He S, Walls AF. The induction of a prolonged increase in microvascular permeability by human mast cell chymase. Eur J Pharmacol. 1998; 352:91–98.
32. He S, Walls AF. Human mast cell chymase induces the accumulation of neutrophils, eosinophils and other inflammatory cells in vivo. Br J Pharmacol. 1998; 125:1491–1500.
33. Tomimori Y, Tsuruoka N, Fukami H, Saito K, Horikawa C, Saito M, Muto T, Sugiura N, Yamashiro K, Sumida M, Kakutani S, Fukuda Y. Role of mast cell chymase in allergen-induced biphasic skin reaction. Biochem Pharmacol. 2002; 64:1187.
34. Natsuaki M, Yano N, Yamaya K, Kitano Y. Immediate contact hypersensitivity induced by repeated hapten challenge in mice. Contact Dermatitis. 2000; 43:267–272.
35. Dearman RJ, Basketter DA, Kimber I. Variable effects of chemical allergens on serum IgE concentration in mice: preliminary evaluation of a novel approach to the identification of respiratory sensitizers. J Appl Toxicol. 1992; 12:317–323.
36. Dearman RJ, Basketter DA, Kimber I. Selective induction of type 2 cytokines following topical exposure of mice to platinum salts. Food Chem Toxicol. 1998; 36:199–207.
37. Kitagaki H, Ono N, Hayakawa K, Kitazawa T, Watanabe K, Shiohara T. Repeated elicitation of contact hypersensitivity induces a shift in cutaneous cytokine milieu from a T helper cell type 1 to a T helper cell type 2 profile. J Immunol. 1997; 159:2484–2491.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download