Abstract
To determine the proportion of nerve fibers in the hypogastric nerve (HGN) and pelvic splanchnic nerve (PSN), small tissue strips of the HGN and PSN from 12 donated elderly cadavers were examined histologically. Immunohistochemistry for neuronal nitric oxide synthase (NOS), vasoactive intestinal peptide (VIP), and tyrosine hydroxylase (TH) was performed. More than 70% of fibers per bundle in the HGN were positive for TH at the level of the sacral promontory. In addition, NOS- (negative) and/or VIP+ (positive) fibers were observed in small areas of each nerve bundle, although the proportion of each was usually less than 10%. In the PSN near the third sacral nerve root, the proportion of nerve fibers positive for NOS and/or VIP (or TH) was below 30%. In both the HGN and PSN, the number of VIP+ fibers was usually greater than that of NOS+ fibers, with frequent co-localization of NOS and VIP. More fibers in both nerves were positive for TH than for these other markers. In contrast to pelvic plexus branches, there were no differences in the proportions of NOS+ and VIP+ fibers between nerve bundles in each of the tissue strips. Thus, target-dependent sorting of nerve fibers was not apparent in the HGN at the level of the sacral promontory or in the PSN near the third sacral nerve root. The NOS+ and/or VIP+ fibers in the HGN were most likely ascending postganglionic fibers to the colon, while those in the PSN root may be preganglionic fibers from Onuf's nucleus.
The hypogastric nerve (HGN) is likely the major source of sympathetic nerves for the human pelvic viscera, while the pelvic splanchnic nerve (PSN) is the major source of parasympathetic nerves [123]. The sympathetic and parasympathetic nerves in the human pelvic plexus or its branches have been distinguished immunohistochemically in specimens surgically removed from adults [45678] and in human fetuses [910]. For example, the HGN in a 17-week-male fetus was found to be weakly positive for the vesicular acetylcholine transporter, a marker of cholinergic or parasympathetic nerves, whereas the PSN in the fetus was strongly positive [9].
To our knowledge, however, few studies have utilized immunohistochemical methods to distinguish among nerve fibers in the HGN and/or PSN of adults. Although one immunohistochemical study assayed the HGN and PSN in male cadaveric specimens, that study did not involve staining composite nerve fibers but concentrated on ganglion cell distribution [11]. Nerve fibers in the upper part of the pelvic plexus near the sacral promontory were examined in surgically obtained specimens (i.e., uterosacral ligaments) [67]. Although the nerves examined in that study most likely contained a major part of the HGN, the latter was not identified during surgery.
Using a set of antibodies against neuronal nitric oxide synthase (NOS), vasoactive intestinal peptide (VIP), and tyrosine hydroxylase (TH), the composite nerve fibers in the pelvic plexus branches in adult cadavers were recently shown to be distinctly heterogeneous [12]. The pelvic plexus branches could be classified into four combinations of autonomic motor nerves: (NOS+, VIP+, TH+), (NOS+, VIP-, TH+), (NOS-, VIP-, TH+), and (NOS-, VIP-, TH-), with the four other possible combinations not observed. Moreover, two adjacent nerves in the plexus were likely to differ. Positivity for NOS has been used to identify the cavernous nerve [13]. Moreover, NOS+ and VIP+ fibers have been shown to co-occur in branches of the pelvic plexus [4567]. In spite of those detailed information of the adult pelvic plexus branches, we are not able to discuss about whether the HGN or PSN gave off the NOS+, VIP+, or TH+ fibers. A simple theory is likely that parasympathetic nerve fibers (NOS+ or VIP+) came from the PSN and sympathetic fibers (TH+) from the HGN. To characterize the nerve fibers in the HGN and PSN and to determine whether there were differences between nerve branches constituting these two nerves, we analyzed adult cadavers using antibodies to NOS, VIP, and TH.
The study was performed in accordance with the provisions of the Declaration of Helsinki 1995 (as revised in Edinburgh 2000). We examined 12 donated cadavers (5 men and 7 women), of mean age 88 years (range, 74-95 years). Causes of death were ischemic heart failure or intracranial bleeding. To ensure that none of the patients had undergone previous surgery, their individual histories were reviewed and all were subjected to macroscopic observation of the abdominopelvic cavity. The cadavers had been donated to the Tokyo Dental College for research and education on human anatomy, and their use for research had been approved by the university ethics committee. The donated cadavers had been fixed by arterial perfusion of 10% v/v formalin solution and stored in 50% v/v ethanol solution for more than 3 months.
Two unilateral tissue blocks, one each containing the HGN and PSN, were obtained from each cadaver. The HGN runs immediately beneath the peritoneum in front of the sacral promontory [14]. This superficial nerve course is easy to identify during open surgery on the abdomen [15] and a surgical plane in front of the HGN is useful for mesorectal incision during rectal surgery [16]. In contrast, the PSN runs deep posterolateral to the rectum. A simple method, involving lateral traction of the pelvic viscera after bisection of the cadaveric pelvis, has been used to identify a connective tissue bundle containing the PSN [17]. Detailed dissection of the PSN has also been accomplished using the same approach after bisection of the pelvis [18]. We therefore used the same method to obtain the third sacral root of the PSN (Fig. 1A, B), which apparently corresponded to the previously described "common nerve root of the PSN" [19]. The tissue strips for the HGN were delineated by the peritoneum anteriorly, while those for the PSN often contained part of the sacrospinous ligament posteriorly (Fig. 1F, G).
Following routine procedures for paraffin-embedded histology, serial cross sections of each of tissue block were prepared. Most sections were stained with hematoxylin and eosin or Masson trichrome, with others used for immunohistochemistry. The primary antibodies used for nerve immunohistochemistry included (1) rabbit polyclonal anti-human NOS (1:200, Cell Signaling Technology, Beverly, MA, USA), (2) mouse monoclonal anti-human VIP (1:100, sc25347, Santa Cruz Biotechnology, Santa Cruz, CA, USA), and (3) rabbit polyclonal anti-human TH (1:100, ab152, Millipore-Chemicon, Temecula, CA, USA) [1220]. Use of these cadaveric materials made immunohistochemical identification of vesicular acetylcholine transporter, neuropeptide Y, calcitonin gene-related peptide and substance P difficult [20]. The samples were subsequently incubated with horseradish peroxidase (HRP)-labeled secondary antibody, with antigen-antibody reactions detected by the HRP-catalyzed reaction with diaminobenzidine. All samples were subsequently counterstained with hematoxylin. The negative control for each specimen consisted of sections of the same sample not reacted with primary antibody. The positive control for each cadaver consisted of the myenteric plexus of the descending colon (Fig. 1C-E), which shows weak reaction with antibody to TH, due to the presence of a few TH+ myenteric neurons, along with densely distributed neuronal NOS/VIP-coreactive nerve fibers, even in centenarians [21].
Samples were observed and photographs taken with a Nikon Eclipse 80, except that photos at ultralow magnification (less than ×1 at the objective lens) were taken using a high-grade flat scanner with translucent illumination (Epson scanner GTX970). In eight of the 12 cadavers, we manually counted (1) the numbers of nerve bundles in the unilateral hypogastric and PSNs at ×2-4 objective; and (2) the numbers of composite nerve fibers per nerve bundle at ×40 objective [22].
At the level of the sacral promontory, the unilateral HGN was composed of 3-21 middle sized bundles, each 0.05-0.2 mm in diameter, and 50 or more thinner nerves (Table 1). The former contained 450-750 nerve fibers per bundle, while the latter contained 40-100 fibers per bundle. Table 2 shows the numbers of nerve fibers in one middle-sized bundle chosen randomly from eight of the 12 cadavers. In addition, seven cadavers carried 1-3 thick nerve bundles, each more than 0.2 mm in diameter and containing more than 4,000-5,000 nerve fibers. The thin nerves tended to concentrate into areas around an arteries, while thick and moderate-sized nerves were distributed in the lateral sites near the ureter. Although we examined only 4-6 nerve bundles in each tissue strip (Fig. 1F), the HGN usually contained abundant, unmyelinated, thin nerve fibers. Based on these findings, we estimated that each unilateral HGN included 6,000-25,000 nerve fibers.
TH+ nerve fibers were consistently abundant in all nerve bundles (Figs. 2, 3, 4), irrespective of the thickness of the nerve bundle and its site along the mediolateral axis. About 70% of the fibers in each nerve bundle were TH+. In contrast, NOS+ and/or VIP+ fibers were located in small areas of each nerve bundle (Fig. 2A, B, D, E). Of these fibers, fewer than 10% were positive for these antigens, except in a 93-year-old man, in whom almost 30% of the fibers were positive (Table 2, Fig. 3A, B, D, E). Most bundles contained more VIP+ than NOS+ fibers, with the distributions of these fibers tending to overlap in each nerve bundle. Differences in the proportions of NOS+ and VIP+ fibers among nerve bundles were observed in each of the tissue strips (Fig. 2), but differences were less than 10%. Two or three ganglion cell clusters in the HGN were seen at the promontory level in three of the 12 cadavers (Fig. 4). Although TH+ cells were dominant, intermingling of VIP+ and/or NOS+ cells was observed. NOS+ cells were often positive for VIP. Surprisingly, in one specimen, a ganglion cell appeared to express both VIP and TH (Fig. 4E, F).
Near the third sacral nerve root, the candidate PSN was composed of 3-9 middle sized bundles, each 0.05-0.2 mm in diameter, and 20-40 thinner nerves (Table 1). The former contained 350-800 nerve fibers per bundle, while the latter contained 30-80 fibers each. Table 2 shows the numbers of composite nerve fibers in the middle-sized nerve bundle. In addition, five cadavers carried 2-4 thick nerve bundles, each more than 0.2 mm in diameter and containing 3,500-6,000 nerve fibers. All these nerves were distributed almost evenly in loose connective tissue. Based on these findings, we estimated that 2,000-25,000 nerve fibers were present in each unilateral PSN.
We examined 3-4 nerve bundles in each tissue strip (Fig. 1G). Although we did not perform myelin sheath staining, such as with Luxor fast blue, the PSNs consistently contained abundant, myelinated, thick nerve fibers. Some of these thick fibers appeared positive for NOS or VIP, but all were negative for TH. There were two groups of nerve bundles: (1) NOS+ or VIP+ fiber-rich bundles of which proportion reached 20%-30% (Fig. 5) and (2) NOS+ or VIP+ fiber-poor bundles (1%-3%), with the latter type observed in only three of the 12 cadavers reason (93M, 76F and 78F in Table 2). Interestingly, the 93-year-old man with abundant NOS+ fibers in the HGN (Fig. 3) had few NOS+ fibers in the PSN (Table 2). About 20%-50% of fibers per bundle were TH+, a proportion higher than that of NOS+ or VIP+ fibers (Table 2, Fig. 5). Differences were observed in the proportions of NOS+ or VIP+ fibers among nerve bundles in each of the tissue strips (Fig. 5), but these differences did not exceed 20%. No ganglion cells were present in any of these PSN specimens. Finally, there were no gender- or age-related differences in the morphologies of the HGN and PSN.
To our knowledge, this study is the first to analyze the proportion of composite fibers in the adult HGN and PSN using immunohistochemical markers for both sympathetic and parasympathetic nerves. Although our findings were only rough estimates, the numbers of nerve fibers transferred through the HGN were greater than those transferred through the thoracic splanchnic nerves, whereas the numbers transferred through the PSN and thoracic splanchnic nerves were similar when compared with a reference [23]. The numbers of composite fibers in the human PSN were similar to those reported for the male rhesus monkey (mean, 16,314±6,334 [24]). Similar to previous findings using fetuses [9], we observed that TH+ sympathetic nerve fibers were dominant in both the PSN and HGN. TH+ fibers in the PSN are thought to originate from the sacral sympathetic trunk (i.e., the sacral splanchnic nerve), while TH+ fibers in the HGN should be postganglionic, arising from the celiac and mesenteric ganglia. However, results showing TH+ ganglion cells interposed along the nerve suggest that preganglionic fibers are also contained within the HGN. In contrast to results observed in the pelvic plexus branches [12], we did not observe a distinct difference in composite nerve fibers between nerve bundles in either the HGN or PSN. Thus, target dependent sorting of nerve fibers seemed not to occur in the HGN at the level of the sacral promontory or in the PSN near the third sacral nerve root.
In contrast to the relatively small number of ganglion cells seen in the present study, previous studies have reported more than 100 ganglion cells along either the HGN and PSN in almost all cadavers examined [1125]. These differences were apparently due to the area of sampling, the accuracy of nerve findings and the sampling site. In these earlier studies, the nerve course was estimated in semiserial sections, including the pelvic viscera and connective tissue around the nerves [1125], whereas we strictly limited the sampling area by minimum dissection to obtain the most proximal course of the nerve. Therefore, the earlier studies may have overestimated the numbers of ganglion cells in the HGN and PSN. However, the tissue strips we used to examine the PSN observation were likely to also contain nerves to the levator ani muscle [18], nerves that also contain TH+ fibers [1226].
Because autonomic motor nerve nuclei in the sacral spinal cord, such as Onuf's nucleus, express NOS but not VIP [2728], preganglionic NOS+ VIP- fibers were likely present in the PSN. However, most of the NOS+ fibers we observed in the PSN were also positive for VIP. Because no ganglion cells were present along the third sacral root of the PSN, the VIP+ fibers we observed in the PSN may be postganglionic fibers from ganglion cells in the pelvic plexus and may go in an upward or downward direction along the sacral sympathetic trunk and/or sacral plexus. Nerves to the lower extremities may not contain NOS+ fibers [29], but, to our knowledge, no previous study has examined VIP+ nerves to the lower extremity.
In contrast to the PSN, NOS+ or VIP+ nerve fibers (cholinergic fibers) in the HGN may originate from (1) lowermost peripheral branches of the vagus nerve; (2) ascending, postganglionic fibers from the pelvic plexus to the left-sided colon; or (3) preganglionic fibers from the thoracic or lumbar spinal nerves to the pelvic plexus [9]. The latter third origin is supported by findings of VIP+ neurons (parasympathetic neurons?) in the sympathetic nerve trunk [3031]. Although this type of paradoxical distribution is often seen in the head and neck [32] the number of candidate cholinergic fibers in the HGN was apparently too large. It is more likely that the NOS+ or VIP+ nerve fibers (cholinergic fibers) in the HGN originate from the ascending fibers. A fourth possibility was suggested by the finding that VIP+ fibers in the pelvic floor may include sensory fibers [333435].
Figures and Tables
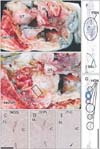 | Fig. 1Tissue sampling to prepare histological sections of the pelvic splanchnic nerve (PSN) from the left hemipelvis of a 78-year-old woman. (A) Picture of the body immediately after bisection of the pelvis. The hypogastric nerve (HGN) was sampled in front of the sacral promontory, and the body was cut transversely along the fifth lumbar vertebra. (B) Dissection of the sacral nerve roots (S1, S2, S3) during lateral traction of the pelvic viscera. The body of the uterus (UT) had been removed between steps shown in panels (A) and (B). The material analyzed, which included the third sacral root of the PSN, was obtained from the area indicated by the square in panel (B). (C-E) Positive controls for the present immunohistochemical assays: serial cross sections of the descending colon of the same specimen for the hypogastric and splanchnic nerves were incubated with antibodies against neuronal nitric oxide synthase (NOS) (C), vasoactive intestinal peptide (VIP) (D), and tyrosine hydroxylase (TH) (E). In contrast to the very restricted expression of TH (arrow in panel E), NOS and VIP were strongly and widely positive in the myenteric plexus (stars in panels C and D). (F, G) Masson trichrome staining of tissue strips containing the HGN (F) and PSN (G). Panels (C-E) are shown at the same magnification as are panel (F) and (G). Scale bars=0.5 mm (C), 10 mm (G). BL, bladder; EL, external longitudinal muscle layer of the colon; IC, internal circular muscle layer of the colon; P, pubis; R, rectum; SSL, sacrospinous ligament; VAG, vagina. |
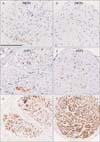 | Fig. 2Nerve bundles comprising the hypogastric nerve in a 78-year-old man. Serial cross-sections of middle-sized bundles (A-C) and a thick bundle (D-F) were incubated with antibodies to neuronal nitric oxide synthase (NOS) (A, D), vasoactive intestinal peptide (VIP) (B, E), and tyrosine hydroxylase (TH) (C, F). The numbers of NOS- and VIP+ fibers were greater in panels (A) and (B) than in panels (D) and (E). In both types of nerve bundles, NOS+ or VIP+ fibers were restricted to small areas and overlapped. TH+ fibers were distributed throughout the nerve bundles. All panels were prepared at the same magnification. Scale bar=0.1 mm (A). |
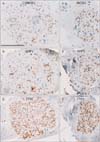 | Fig. 3Hypogastric nerve containing abundant fibers positive for neuronal nitric oxide synthase (NOS) in a 93-year-old man. Serial cross-sections of a middle-sized bundle (A-C) and a thin bundle (D-F) were incubated with antibodies against NOS (A, D), vasoactive intestinal peptide (VIP) (B, E), and tyrosine hydroxylase (TH) (C, F). The distributions of NOS- and VIP+ fibers appeared to overlap, whereas TH+ fibers were distributed throughout the nerve bundles. The hypogastric nerve in this cadaver contained the largest numbers of fibers positive for NOS and VIP of the 12 cadavers examined. All panels were prepared at the same magnification. Scale bar=0.1 mm (A). |
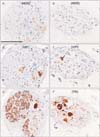 | Fig. 4Hypogastric nerve containing ganglion cells in an 85-year-old woman. Serial sections of two middle-sized bundles, represented by panels (A-C) and panels (D-F), respectively, were incubated with antibodies against neuronal nitric oxide synthase (NOS) (A, D), vasoactive intestinal peptide (VIP) (B, E), and tyrosine hydroxylase (TH) (C, F). The expression of NOS and VIP overlapped in some ganglion cells (A, B), whereas TH+ fibers were distributed throughout the nerve bundles. One ganglion cell (arrow in panels E and F) was apparently positive for both TH and VIP. All panels were prepared at the same magnification. Scale bar=0.1 mm (A). |
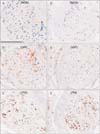 | Fig. 5Nerve bundles constituting the pelvic splanchnic nerve in a 78-year-old man. These were the same specimens as those shown in Fig. 2. Serial cross sections of two middle-sized bundles, represented by panels (A-C) and panels (D-F), respectively, were incubated with antibodies against neuronal nitric oxide synthase (NOS) (A, D), vasoactive intestinal peptide (VIP) (B, E), and tyrosine hydroxylase (TH) (C, F). In panels (A) and (B), the distributions of NOS+ and VIP+ fibers overlapped and differed from the distribution of TH+ fibers. The numbers of NOS- and VIP+ fibers were greater in panels (A) and (B) than in panels (D) and (E). The density of TH+ fibers (C, F) was much lower than in the hypogastric nerve (Fig. 2C, F). All panels were prepared at the same magnification. Scale bar=0.1 mm (A). |
Table 1
Sizes of nerve bundles constructing the unilateral hypogastric or pelvic splanchnic nerve in 8 of the 12 cadavers examined

Table 2
Composite fibers of the hypogastric and pelvic splanchnic nerves

In each of the 8 cadavers, we randomly chose a middle-sized nerve bundle (definition, see Table 1) from the unilateral hypogastric nerve or the pelvic splanchnic nerve.
Number of positive fibers in the immunohistochemistry for neuronal nitric oxide synthase (NOS), vasoactive intestinal peptide (VIP), or tyrosine hydroxylase (TH).
Acknowledgements
We thank those people who donated their bodies after death to Tokyo Dental College for research and education on human anatomy without any socio-economic benefit. We also thank their families for agreeing to these donations and for their patience in waiting for the return of their bones after completion of the study.
References
1. Maas CP, DeRuiter MC, Kenter GG, Trimbos JB. The inferior hypogastric plexus in gynecologic surgery. J Gynecol Tech. 1999; 5:55–62.
2. Baader B, Herrmann M. Topography of the pelvic autonomic nervous system and its potential impact on surgical intervention in the pelvis. Clin Anat. 2003; 16:119–130.
3. Mauroy B, Demondion X, Bizet B, Claret A, Mestdagh P, Hurt C. The female inferior hypogastric (= pelvic) plexus: anatomical and radiological description of the plexus and its afferences--applications to pelvic surgery. Surg Radiol Anat. 2007; 29:55–66.
4. Hoyle CH, Stones RW, Robson T, Whitley K, Burnstock G. Innervation of vasculature and microvasculature of the human vagina by NOS and neuropeptide-containing nerves. J Anat. 1996; 188(Pt 3):633–644.
5. Busacchi P, De Giorgio R, Santini D, Bellavia E, Perri T, Oliverio C, Paradisi R, Corinaldesi R, Flamigni C. A histological and immunohistochemical study of neuropeptide containing somatic nerves in the levator ani muscle of women with genitourinary prolapse. Acta Obstet Gynecol Scand. 1999; 78:2–5.
6. Butler-Manuel SA, Buttery LD, A'Hern RP, Polak JM, Barton DP. Pelvic nerve plexus trauma at radical and simple hysterectomy: a quantitative study of nerve types in the uterine supporting ligaments. J Soc Gynecol Investig. 2002; 9:47–56.
7. Butler-Manuel SA, Buttery LD, Polak JM, A'Hern R, Barton DP. Autonomic nerve trauma at radical hysterectomy: the nerve content and subtypes within the superficial and deep uterosacral ligaments. Reprod Sci. 2008; 15:91–96.
8. Hisasue S, Hashimoto K, Kobayashi K, Takeuchi M, Kyoda Y, Sato S, Masumori N, Tsukamoto T. Baseline erectile function alters the cavernous nerve quantity and distribution around the prostate. J Urol. 2010; 184:2062–2067.
9. Alsaid B, Bessede T, Karam I, Abd-Alsamad I, Uhl JF, Benoit G, Droupy S, Delmas V. Coexistence of adrenergic and cholinergic nerves in the inferior hypogastric plexus: anatomical and immunohistochemical study with 3D reconstruction in human male fetus. J Anat. 2009; 214:645–654.
10. Moszkowicz D, Peschaud F, Bessede T, Benoit G, Alsaid B. Internal anal sphincter parasympathetic-nitrergic and sympathetic-adrenergic innervation: a 3-dimensional morphological and functional analysis. Dis Colon Rectum. 2012; 55:473–481.
11. Takenaka A, Kawada M, Murakami G, Hisasue S, Tsukamoto T, Fujisawa M. Interindividual variation in distribution of extramural ganglion cells in the male pelvis: a semi-quantitative and immunohistochemical study concerning nerve-sparing pelvic surgery. Eur Urol. 2005; 48:46–52.
12. Hinata N, Hieda K, Sasaki H, Murakami G, Abe S, Matsubara A, Miyake H, Fujisawa M. Topohistology of sympathetic and parasympathetic nerve fibers in branches of the pelvic plexus: an immunohistochemical study using donated elderly cadavers. Anat Cell Biol. 2014; 47:55–65.
13. Yucel S, De Souza A Jr, Baskin LS. Neuroanatomy of the human female lower urogenital tract. J Urol. 2004; 172:191–195.
14. Tamakawa M, Murakami G, Takashima K, Kato T, Hareyama M. Fascial structures and autonomic nerves in the female pelvis: a study using macroscopic slices and their corresponding histology. Anat Sci Int. 2003; 78:228–242.
15. Maas K, Moriya Y, Kenter G, Trimbos B, van de Velde C. A plea for preservation of the pelvic autonomic nerves. Lancet. 1999; 354:772–773.
16. Kinugasa Y, Murakami G, Suzuki D, Sugihara K. Histological identification of fascial structures posterolateral to the rectum. Br J Surg. 2007; 94:620–626.
17. Kinugasa Y, Murakami G. The contents of lateral ligaments: is organized connective tissue present? Dis Colon Rectum. 2006; 49:1243–1244.
18. Takeyama M, Koyama M, Murakami G, Nagata I, Tomoe H, Furuya K. Nerve preservation in tension-free vaginal mesh procedures for pelvic organ prolapse: a cadaveric study using fresh and fixed cadavers. Int Urogynecol J Pelvic Floor Dysfunct. 2008; 19:559–566.
19. Akita K, Sakamoto H, Sato T. Origins and courses of the nervous branches to the male urethral sphincter. Surg Radiol Anat. 2003; 25:387–392.
20. Hieda K, Cho KH, Arakawa T, Fujimiya M, Murakami G, Matsubara A. Nerves in the intersphincteric space of the human anal canal with special reference to their continuation to the enteric nerve plexus of the rectum. Clin Anat. 2013; 26:843–854.
21. Bernard CE, Gibbons SJ, Gomez-Pinilla PJ, Lurken MS, Schmalz PF, Roeder JL, Linden D, Cima RR, Dozois EJ, Larson DW, Camilleri M, Zinsmeister AR, Pozo MJ, Hicks GA, Farrugia G. Effect of age on the enteric nervous system of the human colon. Neurogastroenterol Motil. 2009; 21:746–e46.
22. Nakao T, Cho KH, Yamamoto M, Yamane S, Murakami G, Ide Y, Abe S. Site-dependent difference in the density of sympathetic nerve fibers in muscle-innervating nerves: a histologic study using human cadavers. Eur J Anat. 2012; 16:33–42.
23. Kuntz A, Hoffman H, Schaeffer EM. Fiber components of the splanchnic nerves. Anat Rec. 1957; 128:139–146.
24. Schnitzlein HN, Hoffman HH, Tucker CC, Quigley MB. The pelvic splanchnic nerves of the male Rheusus monkey. J Comp Neurol. 1960; 114:51–65.
25. Imai K, Furuya K, Kawada M, Kinugasa Y, Omote K, Namiki A, Uchiyama E, Murakami G. Human pelvic extramural ganglion cells: a semiquantitative and immunohistochemical study. Surg Radiol Anat. 2006; 28:596–605.
26. Hinata N, Hieda K, Sasaki H, Kurokawa T, Miyake H, Fujisawa M, Murakami G, Fujimiya M. Nerves and fasciae in and around the paracolpium or paravaginal tissue: an immunohistochemical study using elderly donated cadavers. Anat Cell Biol. 2014; 47:44–54.
27. Gibson SJ, Polak JM, Katagiri T, Su H, Weller RO, Brownell DB, Holland S, Hughes JT, Kikuyama S, Ball J, Bloom SR, Steiner TJ, de Belleroche J, Clifford Rose F. A comparison of the distributions of eight peptides in spinal cord from normal controls and cases of motor neurone disease with special reference to Onuf's nucleus. Brain Res. 1988; 474:255–278.
28. Pullen AH, Humphreys P, Baxter RG. Comparative analysis of nitric oxide synthase immunoreactivity in the sacral spinal cord of the cat, macaque and human. J Anat. 1997; 191(Pt 2):161–175.
29. Hosaka F, Katori Y, Kawase T, Fujimiya M, Ohguro H. Site-dependent differences in density of sympathetic nerve fibers in muscle-innervating nerves of the human head and neck. Anat Sci Int. 2014; 89:101–111.
30. Lindh B, Lundberg JM, Hökfelt T. NPY-, galanin-, VIP/PHI-, CGRP- and substance P-immunoreactive neuronal subpopulations in cat autonomic and sensory ganglia and their projections. Cell Tissue Res. 1989; 256:259–273.
31. Roudenok V, Kuhnel W, Rogov Y, Nerovnja A. Developmental changes in vasoactive intestinal polypeptide immunoreactivity in the human paravertebral ganglia. Ann Anat. 1999; 181:561–565.
32. Kiyokawa H, Katori Y, Cho KH, Murakami G, Kawase T, Cho BH. Reconsideration of the autonomic cranial ganglia: an immunohistochemical study of mid-term human fetuses. Anat Rec (Hoboken). 2012; 295:141–149.
33. Maggi CA, Giuliani S, Santicioli P, Patacchini R, Said SI, Theodorsson E, Turini D, Barbanti G, Giachetti A, Meli A. Direct evidence for the involvement of vasoactive intestinal polypeptide in the motor response of the human isolated ileum to capsaicin. Eur J Pharmacol. 1990; 185:169–178.
34. Jacobson ED, Berguer R, Pawlik WW, Hottenstein OD. Mesenteric purinergic and peptidergic vasodilators. In : Johnson LR, Alpers DH, Christensen J, Jacobson ED, Walsh JH, editors. Physiology of the Gastrointestinal Tract. New York: Raven Press;1994. p. 1647–1667.
35. Kawatani M, Suzuki T, de Groat WC. Corticotropin releasing factor-like immunoreactivity in afferent projections to the sacral spinal cord of the cat. J Auton Nerv Syst. 1996; 61:218–226.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download