Abstract
Reciprocal exchange of morphogenetic proteins between epithelial and mesenchymal cells in a stem/progenitor cell niche results in formation of a nephron. To maintain diffusion of morphogenetic proteins, it is assumed that a close contact exists between involved cells. However, recent publications underline that both types of stem/progenitor cells are separated by a striking interface. To explore this microarchitecture in detail, neonatal rabbit kidneys were fixed in traditional glutaraldehyde (GA) solution for transmission electron microscopy. For contrast enhancing specimens were fixed in GA solution including cupromeronic blue, ruthenium red or tannic acid. To record same perspectives, embedded blocks of parenchyma were cut in exactly orientated vertical and transverse planes to lining collecting ducts. Electron microscopy of specimens fixed by traditional GA solution illustrates a spatial separation of stem/progenitor cells and an unobstrusively looking interface. In contrast, advanced fixation of specimens in GA solution including cupromeronic blue, ruthenium red and tannic acid unmasks earlier not visible extracellular matrix. In addition, projections of mesenchymal cells covered by matrix cross the interface to contact epithelial cells. Surprisingly, the end of a mesenchymal cell projection does not dangle but is enclosed in a fitting sleeve and connected via tunneling nanotubes with the plasma membrane of an epithelial cell. Regarding this complex ensemble the question is to what extent illustrated cell-cell connections and extracellular matrix are involved in communication and transmission of morphogenetic proteins during induction of a nephron.
Spatial growth of a mammalian kidney depends on a cell biological process called branching morphogenesis. It starts with the organ anlage and ends after induction of the last nephron, when a kidney has reached its final size [1]. Extension of parenchyma is further based on a specific spatiotemporal program conducted by numerous stem/progenitor cell niches each containing cells of the metanephric mesenchyme and epithelial cells [2, 3]. The occurrence of epithelial stem/progenitor cells is special, since they are concentrated during anlage of a kidney in the ureteric bud, while during subsequent radial growth of parenchyma they are recognized in the tip of a bud-derived collecting duct (CD) ampulla [4, 5]. In contrast, mesenchymal stem/progenitor cells are grouped around the tip of an ampulla so that they are always exposed to the basal aspect of epithelial cells [6]. Thus, the peculiar arrangement of both epithelial and mesenchymal stem/progenitor cells constitutes the niche that stays during kidney development as an ensemble and in an always close spatial relationship with the inner side of the organ capsule [7].
Formation of a nephron will start, when the dichotomous arborisation of an ureteric bud respectively the tip of a CD ampulla is fulfilled [8]. Yet epithelial and mesenchymal stem/progenitor cells stand in the correct position for a reciprocal exchange of a series of morphogenetic proteins such as glial-derived neurotrophic factor, hepatocyte growth factor, epidermal growth factor (EGF) receptor ligands (EGF, HBEGF, tumor growth factor [TGF] α), WNT family members, bone morphogenetic proteins, TGFβ, fibroblast growth factors, and leukemia inhibitory factor [9, 10]. As a result first separation and then condensation of few elected mesenchymal cells takes place forming in turn a renal vesicle as the first visible sign of a nephron anlage. Although numerous literature is available about involved morphogenetic proteins, surprisingly little attention was paid to closely associated questions such as their secretion and diffusion course to related receptors within the original niche environment [10].
In the last years it was demonstrated that the renal stem/progenitor cell niche is not an incidental conglomerate but accommodates cells within a structured extracellular matrix [11,12,13]. Although strongly involved in the transmission of morphogenetic proteins, epithelial cells do not stand naked but are covered at their basal aspect by a noticable basal lamina [14]. In close spatial relation microfibers consisting of collagen type I, II, III, and IV arise [7, 15]. Further on, Soybean agglutinin-labeled microfibers originate at the basal lamina to pass through the group of mesenchymal cells up to the organ capsule [16]. Thus, the occurrence of microfibers elucidates that cells within the renal stem/progenitor cell niche are domiciled within an unexpected wire netting. More over, the fastening of the renal stem/progenitor cell niche by diverse microfibers at the organ capsule explains its constant presence in the cortex corticis throughout organ expansion during development [7].
As well previous investigations as here presented images of human (Fig. 1B) and rabbit (Fig. 1C) niches taken by optical microscopy demonstrate in a vertical perspective that epithelial and mesenchymal stem/progenitor cells do not touch but stand at a distance between 1 to 2 µm [17, 18]. Previously, it was argued that the illustrated gap reflects an artifact caused by poor preparation. However, cryosections, paraffine and resin embedded embryonic parenchyma of carefully fixed specimens reveal that the gap between both types of stem/progenitor cells is constant. Further on, vertically orientated blocks of parenchyma (Fig. 1D) can be turned at a right angle so that a transversal view on different planes of neighboring niches becomes possible (Fig. 1E). For example, when section planes 1, 2, 3, or 4 (Fig. 1D) are compared, epithelial cells within a CD ampulla are seen that are separated from surrounding mesenchymal cells by a constant and clearly visible gap (Fig. 1E).
There must be a reason for the spatial separation of epithelial and mesenchymal stem/progenitor cells. When an artifact is excluded, the gap between epithelial and mesenchymal cells within the renal niche must be caused either by interstitial fluid and/or by masked extracellular matrix. To find a morphological correlate, analysis by transmission electron microscopy seemed to be the adequate technique. However, specimens fixed in traditional glutaraldehyde (GA) solution did not provide new evidence. For that reason alternative fixation was performed by GA solution including either 0.1% cupromeronic blue, 0.5% ruthenium red or 1% tannic acid [19].
As a first step transversal sections of the renal stem/progenitor cell niche were screened in the electron microscope under low enlargement (Fig. 2). It can be seen that epithelial cells are enclosed in the tip of a CD ampulla but in each of analyzed cases they are separated from mesenchymal cells. The gap in-between looks bright and unobstrusive, when fixation of specimens is performed by traditional GA solution (Fig. 2B), GA solution including cupromeronic blue (Fig. 2C) or ruthenium red (Fig. 2D). Surprisingly, when fixation is performed in GA solution including tannic acid (Fig. 2E), beside the gap a band of labeled extracellular matrix at the basal aspect of epithelial cells is visible.
Electron microscopy under middle enlargement illustrates that the body of mesenchymal cell has almost the same distance to the basal lamina of an opposite epithelial cell (Fig. 3). This result is a clear hint that the gap earlier seen in optical microscopy must be based on a structured interface. However, specimens fixed in traditional GA solution exhibit that a mesenchymal cell body is separated from epithelial cells by an interface that looks bright and unobtrusive (Fig. 3B). Further epithelial cells at the tip of a CD ampulla are covered by a basal lamina consisting of a lamina rara, lamina densa, and lamina fibroreticularis. Occasionally, tiny microfibers originate at the lamina fibroreticularis and span through the interface to touch mesenchymal cells.
Surprisingly, projections of mesenchymal cells do not dangle in the interface but cross it to contact the basal lamina at the opposite epithelial cell. Specimens fixed in GA solution including cupromeronic blue (Fig. 3C) create the same impression of the interface as seen before (Fig. 3B). However, within the basal lamina of epithelial cells and on the surface of contacting mesenchymal cell projections numerous braces of proteoglycans become visible as a punctuate pattern. Moreover, specimens fixed in GA solution including ruthenium red (Fig. 3D) or tannic acid (Fig. 3E) show an intense label covering the basal lamina of epithelial cells and to a high degree contacting projections of mesenchymal cells. Label by tannic acid further shows that a network of small dark spots is widely distributed in the interface. Thus, electron microscopy clearly demonstrates that textural extracellular matrix labeled by ruthenium red (Fig. 3D) and tannic acid (Fig. 3E) covers epithelial and mesenchymal cells including projections. This construction acts as a corset spacer and leads in turn to the illustrated interface.
Finally, since all of the morphological details are well preserved and label is not smearing, recorded micrographs reflect the natural situation and cannot be described as an artifact.
Of special interest is, whether the endings of mesenchymal cell projections establish a functional connection between the two types of stem/progenitor cells [20]. High magnification of samples fixed in traditional GA solution demonstrates that the end of a mesenchymal cell projection is touching the lamina fibroreticularis at the tip of a CD ampulla (Fig. 4B, F). At the contact site the plasma membrane appears to be compacted providing the impression that only a short lasting connection exists (Fig. 4B). In contrast, fixation in GA solution including cupromeronic blue shows numerous braces of proteoglycans that originate at the basal lamina of epithelial cells to form a sleeve around the surface of a contacting mesenchymal cell projections (Fig. 4C, G). This unexpected construction points out that the contact between a mesenchymal cell projection and an epithelial cell is not random but constant. Further on, fixation of specimens by GA solution including ruthenium red (Fig. 4D, H) or tannic acid (Fig. 4E, I) illustrates earlier non visible structures. In both series can be seen that the basal lamina of epithelial cells within the tip of a CD ampulla is covered by a striking band of textural extracellular matrix. In contrast to traditional fixation (Fig. 4B), substructures such as the lamina rara, lamina densa and lamina fibroreticularis are yet barely visible. However, the end of a mesenchymal cell projection is surrounded by illustrated extracellular matrix forming a special sleeve. Label by ruthenium red (Fig. 4D) and tannic acid (Fig. 4E) further illustrates that tunneling nanotubes are present to create a functional connection between mesenchymal and epithelial stem/progenitor cells.
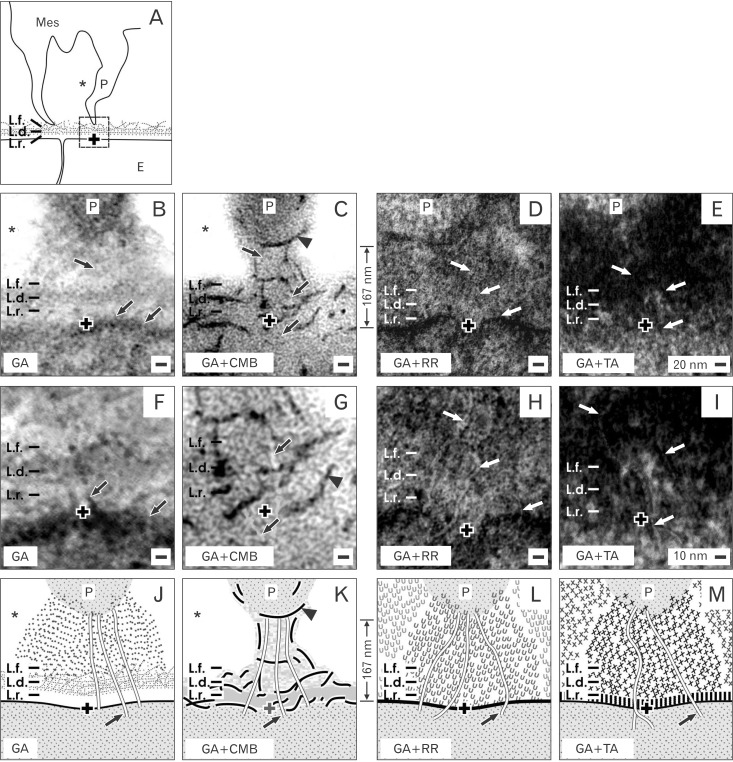
Thus, there is no longer any doubt that the end of a mesenchymal cell projection first contacts the lamina fibroreticularis and forms then a special junction in the lamina densa and rara of epithelial cells via tunneling nanotubes. Very high enlargement in the electron microscope further elucidates that the contact site has a special construction. Interestingly, in all of analyzed samples the distance between the end of a mesenchymal cell projection and the plasma membrane of an epithelial cell is constant and in average 167 nm (Fig. 5). As observed after traditional fixation by GA solution, the end of a mesenchymal cell projection is mounted on a cone-shaped pedestal consisting of fuzzy extracellular matrix (Fig. 5B). Fixation of specimens by GA solution including cupromeronic blue demonstrates that the end of a mesenchymal cell projection is held in position by numerous braces of proteoglycans forming a cylindrical sleeve (Fig. 5C). Fixation of specimens in GA solution including ruthenium red (Fig. 5D) or tannic acid (Fig. 5E) illuminates that the contact zone is concealed by an unexpected amount of micro-structured extracellular matrix. Finally, tunneling nanotubes are recognized that originate at the end of a mesenchymal cell projection, cross the basal lamina to connect the basal plasma membrane of an epithelial cell (Fig. 5F-I). Thus, the data exhibit that illustrated contacts are not incidental, but resemble functional plug-in connections as it is explained in schematic illustrations (Fig. 5J-M). To the best of our knowledge, comparable structures have not been previously displayed.
To move presented morphological data to a cell biological understanding, one can assume that at the end of a mesenchymal cell projection integrin α8β1 is localized, which contacts nephronectin as receptor on the basal lamina of epithelial cells [21, 22, 23]. Further kinesin KIF26B might be present in mesenchymal cell projections possibly involved in regulating the attraction of epithelial stem/progenitor cells [24, 25]. However, presently performed immunohistochemistry in our laboratory with related antibodies did not show clear evidence so that this hypothesis cannot yet be confirmed.
Moreover, it was shown that tunneling nanotubes are generally involved in the intercellular transfer of organelles, membrane compounds and cytoplasmic molecules [26, 27]. However, the actual situation is that tunneling nanotubes were not earlier described for the embryonic and adult kidney, but were solely investigated on renal cell cultures [28, 29]. For that reason general data about occurrence, course, construction and function of tunneling nanotubes in the embryonic kidney including the niche are lacking. Another problem is that specific antibodies for tunneling nanotubes are up to date not available so that appropriate tracer and transport experiments on living renal niches cannot yet be performed. Thus, the challenge for the future is to label organelles or molecules to register their transport between mesenchymal and epithelial stem/progenitor cells via tunneling nanotubes.
Only speculations can be made about the functions of here illustrated tunneling nanotubes within the renal stem/progenitor cell niche (Figs. 4D, E, 5). It is generally accepted that morphogenetic proteins are transmitted for nephron induction between epithelial and mesenchymal stem/progenitor cells via diffusion [9, 30]. However, regarding the here presented results the open question is, whether transmission of morphogenetic proteins takes place exclusively by diffusion or whether also illustrated tunneling nanotubes and cell-cell connections are involved. Such a consideration was up to date neglected. But evidence for such a principle was elaborated decades ago on embryonic mouse kidney and transfilter tubule induction experiments so that it gains new significance by here presented data [31, 32]. Finally, could it be that mesenchymal cells secrete for example morphogenetic proteins via projections and illustrated cell-cell connections, while epithelial cells do it by diffusion (Fig. 5). At present it seems also probable that epithelial cells secrete related morphogenetic proteins via illustrated cell-cell connections.
It is said since long time that isolated stem/progenitor cells are survival and performance artists. However, the present data signal at least for the renal niche that these capabilities are not exclusively contributed to a single cell but must also depend on illustrated extracellular matrix and cell-cell connections (Figs. 3, 4, 5). In turn, the combination of a special microarchitecture, textural extracellular matrix and cell-cell connections does not illustrate lonely fighters but an unexpected functional ensemble within the renal stem/progenitor cell niche.
Of special interest is the question, why contained stem/progenitor cells are no eremites as frequently interpreted but form sophisticated cell-cell connections via tunneling nanotubes so that an earlier not known epithelial-mesenchymal communication is established. Regarding further this ensemble, it becomes probable that essential tasks such as maintenance of stemness, induction of the nephron and control of proliferation/differentiation during condensation of the renal vesicle are regulated here not simply by diffusion of molecules but by sophisticated cell-cell communication. This aspect may be of particular interest for regenerative medicine, since at a special point of the neonatal development formation of nephrons is terminated. In parallel morphological signs of the niche are lost. As a consequence, the process of down-regulation of those niche functions is of outmost importance to learn about and to turn it back for restoration of diseased parenchyma.
Further on, cell sociality and adapted environment is gaining more and more in importance, when implantation of isolated stem/progenitor cells is performed for the repair of diseased renal parenchyma [33, 34]. However, the situation is that a real breakthrough in this kind of therapy was up to date not achieved. One of the major obstacles is that the survival of implanted cells is limited [35]. Thus, learning from nature will help to solve this problem for example by co-implanting stem/progenitor cells with decellularized extracellular matrix derived from niche material [36, 37]. Regarding newly detected extracellular matrix within the renal stem/progenitor cell niche, it justifies to presume that up to date unrecognized cell biological capabilities are contained. The combination with extracellular matrix may help to survive and may stimulate cell competence and its secretome for regeneration of diseased renal parenchyma [38].
Acknowledgements
The authors thank the Department of Molecular and Cellular Anatomy, University of Regensburg for financial support and technical assistance.
References
1. Meyer TN, Schwesinger C, Bush KT, Stuart RO, Rose DW, Shah MM, Vaughn DA, Steer DL, Nigam SK. Spatiotemporal regulation of morphogenetic molecules during in vitro branching of the isolated ureteric bud: toward a model of branching through budding in the developing kidney. Dev Biol. 2004; 275:44–67. PMID: 15464572.
2. Datta A, Bryant DM, Mostov KE. Molecular regulation of lumen morphogenesis. Curr Biol. 2011; 21:R126–R136. PMID: 21300279.
3. Blake J, Rosenblum ND. Renal branching morphogenesis: morphogenetic and signaling mechanisms. Semin Cell Dev Biol. 2014; 36:2–12. PMID: 25080023.
4. Fanos V, Loddo C, Puddu M, Gerosa C, Fanni D, Ottonello G, Faa G. From ureteric bud to the first glomeruli: genes, mediators, kidney alterations. Int Urol Nephrol. 2015; 47:109–116. PMID: 25201458.
5. Nishinakamura R. Stem cells in the embryonic kidney. Kidney Int. 2008; 73:913–917. PMID: 18200005.
6. Kopan R, Chen S, Little M. Nephron progenitor cells: shifting the balance of self-renewal and differentiation. Curr Top Dev Biol. 2014; 107:293–331. PMID: 24439811.
7. Minuth WW, Denk L. Structural links between the renal stem/progenitor cell niche and the organ capsule. Histochem Cell Biol. 2014; 141:459–471. PMID: 24429831.
8. Carroll TJ, Das A. Defining the signals that constitute the nephron progenitor niche. J Am Soc Nephrol. 2013; 24:873–876. PMID: 23578945.
9. Chai OH, Song CH, Park SK, Kim W, Cho ES. Molecular regulation of kidney development. Anat Cell Biol. 2013; 46:19–31. PMID: 23560233.
10. O'Brien LL, McMahon AP. Induction and patterning of the metanephric nephron. Semin Cell Dev Biol. 2014; 36:31–38. PMID: 25194660.
11. Kanwar YS, Wada J, Lin S, Danesh FR, Chugh SS, Yang Q, Banerjee T, Lomasney JW. Update of extracellular matrix, its receptors, and cell adhesion molecules in mammalian nephrogenesis. Am J Physiol Renal Physiol. 2004; 286:F202–F215. PMID: 14707006.
12. Kim HY, Nelson CM. Extracellular matrix and cytoskeletal dynamics during branching morphogenesis. Organogenesis. 2012; 8:56–64. PMID: 22609561.
13. Nigam SK, Bush KT. Growth factor-heparan sulfate "switches" regulating stages of branching morphogenesis. Pediatr Nephrol. 2014; 29:727–735. PMID: 24488503.
14. Strehl R, Minuth WW. Partial identification of the mab (CD)Amp1 antigen at the epithelial-mesenchymal interface in the developing kidney. Histochem Cell Biol. 2001; 116:389–396. PMID: 11735003.
15. Schumacher K, Strehl R, Minuth WW. Characterization of micro-fibers at the interface between the renal collecting duct ampulla and the cap condensate. Nephron Exp Nephrol. 2003; 95:e43–e54. PMID: 14610328.
16. Schumacher K, Strehl R, De Vries U, Groene HJ, Minuth WW. SBA-positive fibers between the CD ampulla, mesenchyme, and renal capsule. J Am Soc Nephrol. 2002; 13:2446–2453. PMID: 12239233.
17. Minuth WW, Denk L, Miess C, Glashauser A. Peculiarities of the extracellular matrix in the interstitium of the renal stem/progenitor cell niche. Histochem Cell Biol. 2011; 136:321–334. PMID: 21822715.
18. Minuth WW, Denk L. Illustration of extensive extracellular matrix at the epithelial-mesenchymal interface within the renal stem/progenitor cell niche. BMC Clin Pathol. 2012; 12:16. PMID: 23009620.
19. Minuth WW, Denk L. Advanced fixation for transmission electron microscopy unveils special extracellular matrix within the renal stem/progenitor cell niche. Methods Mol Biol. 2014; 7. 26. [Epub]. http://dx.doi.org/10.1007/7651_2014_93.
20. Minuth WW, Denk L. Cell projections and extracellular matrix cross the interstitial interface within the renal stem/progenitor cell niche: accidental, structural or functional cues? Nephron Exp Nephrol. 2012; 122:131–140. PMID: 23735962.
21. Muller U, Wang D, Denda S, Meneses JJ, Pedersen RA, Reichardt LF. Integrin alpha8beta1 is critically important for epithelial-mesenchymal interactions during kidney morphogenesis. Cell. 1997; 88:603–613. PMID: 9054500.
22. Brandenberger R, Schmidt A, Linton J, Wang D, Backus C, Denda S, Müller U, Reichardt LF. Identification and characterization of a novel extracellular matrix protein nephronectin that is associated with integrin alpha8beta1 in the embryonic kidney. J Cell Biol. 2001; 154:447–458. PMID: 11470831.
23. Sato Y, Shimono C, Li S, Nakano I, Norioka N, Sugiura N, Kimata K, Yamada M, Sekiguchi K. Nephronectin binds to heparan sulfate proteoglycans via its MAM domain. Matrix Biol. 2013; 32:188–195. PMID: 23357641.
24. Uchiyama Y, Sakaguchi M, Terabayashi T, Inenaga T, Inoue S, Kobayashi C, Oshima N, Kiyonari H, Nakagata N, Sato Y, Sekiguchi K, Miki H, Araki E, Fujimura S, Tanaka SS, Nishinakamura R. Kif26b, a kinesin family gene, regulates adhesion of the embryonic kidney mesenchyme. Proc Natl Acad Sci U S A. 2010; 107:9240–9245. PMID: 20439720.
25. Nishinakamura R, Uchiyama Y, Sakaguchi M, Fujimura S. Nephron progenitors in the metanephric mesenchyme. Pediatr Nephrol. 2011; 26:1463–1467. PMID: 21336811.
26. Kimura S, Hase K, Ohno H. The molecular basis of induction and formation of tunneling nanotubes. Cell Tissue Res. 2013; 352:67–76. PMID: 23229356.
27. Austefjord MW, Gerdes HH, Wang X. Tunneling nanotubes: diversity in morphology and structure. Commun Integr Biol. 2014; 7:e27934. PMID: 24778759.
28. Plotnikov EY, Khryapenkova TG, Galkina SI, Sukhikh GT, Zorov DB. Cytoplasm and organelle transfer between mesenchymal multipotent stromal cells and renal tubular cells in co-culture. Exp Cell Res. 2010; 316:2447–2455. PMID: 20599955.
29. Domhan S, Ma L, Tai A, Anaya Z, Beheshti A, Zeier M, Hlatky L, Abdollahi A. Intercellular communication by exchange of cytoplasmic material via tunneling nano-tube like structures in primary human renal epithelial cells. PLoS One. 2011; 6:e21283. PMID: 21738629.
30. Lander AD. Morpheus unbound: reimagining the morphogen gradient. Cell. 2007; 128:245–256. PMID: 17254964.
31. Lehtonen E. Epithelio-mesenchymal interface during mouse kidney tubule induction in vivo. J Embryol Exp Morphol. 1975; 34:695–705. PMID: 1240119.
32. Lehtonen E, Wartiovaara J, Nordling S, Saxén L. Demonstration of cytoplasmic processes in Millipore filters permitting kidney tubule induction. J Embryol Exp Morphol. 1975; 33:187–203. PMID: 1151265.
33. Fleig SV, Humphreys BD. Rationale of mesenchymal stem cell therapy in kidney injury. Nephron Clin Pract. 2014; 127:75–80. PMID: 25343826.
34. Herrera M, Mirotsou M. Stem cells: potential and challenges for kidney repair. Am J Physiol Renal Physiol. 2014; 306:F12–F23. PMID: 24197069.
35. Burst V, Putsch F, Kubacki T, Völker LA, Bartram MP, Müller RU, Gillis M, Kurschat CE, Grundmann F, Müller-Ehmsen J, Benzing T, Teschner S. Survival and distribution of injected haematopoietic stem cells in acute kidney injury. Nephrol Dial Transplant. 2013; 28:1131–1139. PMID: 23197679.
36. O'Neill JD, Freytes DO, Anandappa AJ, Oliver JA, Vunjak-Novakovic GV. The regulation of growth and metabolism of kidney stem cells with regional specificity using extracellular matrix derived from kidney. Biomaterials. 2013; 34:9830–9841. PMID: 24074840.
37. Yu YL, Shao YK, Ding YQ, Lin KZ, Chen B, Zhang HZ, Zhao LN, Wang ZB, Zhang JS, Tang ML, Mei J. Decellularized kidney scaffold-mediated renal regeneration. Biomaterials. 2014; 35:6822–6828. PMID: 24855960.
38. Tran C, Damaser MS. Stem cells as drug delivery methods: application of stem cell secretome for regeneration. Adv Drug Deliv Rev. 2014; 10. 15. [Epub]. http://dx.doi.org/10.1016/j.addr.2014.10.007.
Fig. 1
(A-E) Vertical (B-D) and transversal (E) views to the renal stem/progenitor cell niche. A paraffin section of fetal human (B) and a semithin section of neonatal rabbit kidney (C) demonstrate an ureteric bud derived collecting duct (CD) ampulla (A) enclosing epithelial cells. Mesenchymal cells are separated by a gap (asterisks). A niche is covered by an organ capsule (C), while at the lateral sides a renal vesicle and a forming S-shaped body are recognized. (D) Schematic illustration shows vertical sections 1 to 4 of the niche. (E) In a semithin section the different planes exhibit that epithelial cells are separated from mesenchymal cells by a gap (asterisks). Human kidney (gestational age between week 16 and 18) was obtained from the stock of preparations used for the Course of Microscopic Anatomy for medical students at the University of Regensburg, Germany.
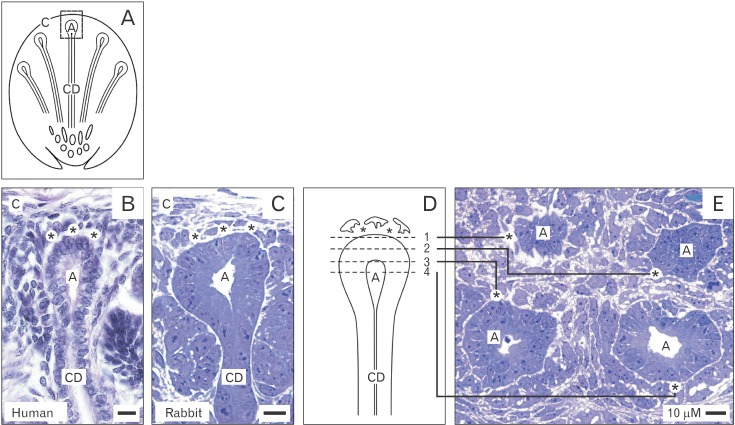
Fig. 2
(A-E) Transversal view to the stem/progenitor cell niche of neonatal rabbit kidney by transmission electron microscopy. Specimens were fixed in traditional glutaraldehyde (GA) solution (B) or GA solution including cupromeronic blue (CMB) (C), ruthenium red (RR) (D), or tannic acid (TA) (E). Epithelial cells enclosed in a collecting duct ampulla (A) are separated from mesenchymal cells by a gap (asterisks). (E) Fixation in GA solution including TA elucidates that in the gap special extracellular matrix is contained.
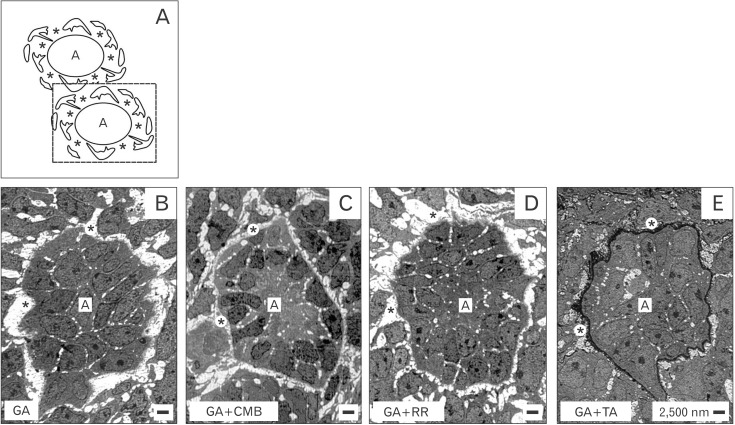
Fig. 3
(A-E) Analysis of the interface within the stem/progenitor cell niche of neonatal rabbit kidney by transmission electron microscopy. Specimens were fixed by traditional glutaraldehyde (GA) solution (B) and GA solution including cupromeronic blue (CMB) (C), ruthenium red (RR) (D), or tannic acid (TA) (E). It can be seen that the distance between mesenchymal and epithelial cells is constant so that both are separated by a striking interface (asterisks). Further projections (P) of mesenchymal cells cross the interface to contact the basal lamina of epithelial cells enclosed within a collecting duct (CD) ampulla. (B) Fixation by GA solution informs that the interface (asterisk) looks bright. (C) Fixation by GA solution including CMB points out that crossing cell projections and the basal lamina of epithelial cells are covered by a punctuate pattern of proteoglycans (arrowhead). (D) Fixation by GA solution including RR unveils a band of extracellular matrix covering the basal lamina of epithelial cells. Cell projections of mesenchymal cells are partially wrapped by this dense label. (E) Fixation by GA solution including TA shows intense label on the basal lamina of epithelial cells. Mesenchymal cell projections are covered by TA label. The basal lamina at the tip of a CD ampulla consists of a lamina rara (L.r.), lamina densa (L.d.) and lamina fibroreticularis (L.f.). The basal plasma membrane of an enclosed epithelial cell is marked by a cross (+).
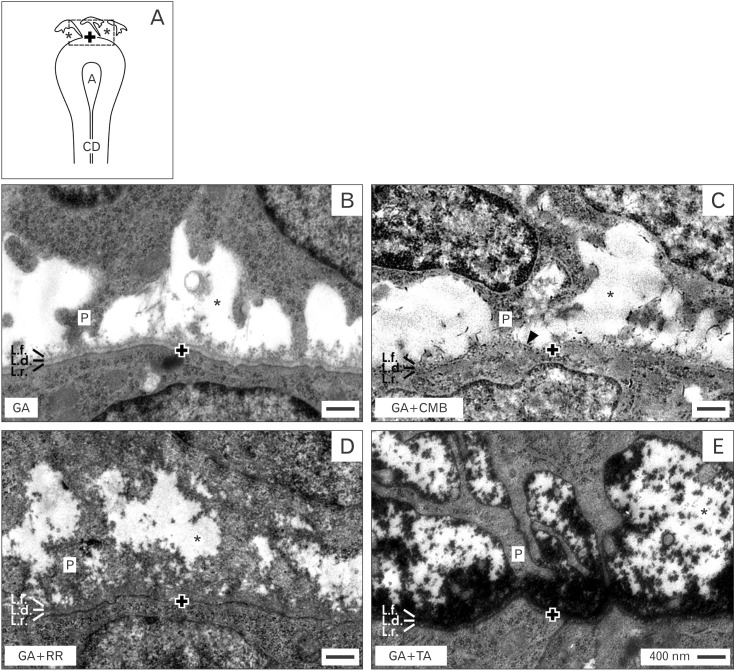
Fig. 4
(A-I) Analysis of mesenchymal cell projections contacting epithelial cells within the stem/progenitor cell niche of neonatal rabbit kidney by transmission electron microscopy. Mes, mesenchymal cell; E, epithelial cell. Specimens were fixed by traditional glutaraldehyde (GA) solution (B, F) and GA solution including cupromeronic blue (CMB) (C, G), ruthenium red (RR) (D, H), or tannic acid (TA) (E, I). (B-E) A mesenchymal cell projection (P) is crossing the interface (asterisks) to contact the basal lamina of epithelial cells. (B) Fixation in GA solution shows that the end of a projection has a tender contact on the basal lamina via microfibers. (C) Fixation in GA solution including CMB informs that a cell projection is mounted by proteoglycan braces (arrowhead). (D) Fixation in GA solution including RR elucidates that a projection is covered by a sleeve of filigree extracellular matrix. (E) Fixation in GA solution including TA demonstrates that a projection is fastened by a labeled sleeve. (D, E) In the center of a projection (marked area) tunneling nanotubes (arrows) are present connecting mesenchymal with epithelial cells. Traditional fixation does not (F), but improved fixation elucidates in a transversal perspective that a mesenchymal cell projection does not dangle but is enclosed by extracellular matrix (G-I). The basal lamina at the tip of a collecting duct ampulla consists of a lamina rara (L.r.), lamina densa (L.d.) and lamina fibroreticularis (L.f.). The basal plasma membrane of enclosed epithelial cell is marked by a cross (+).
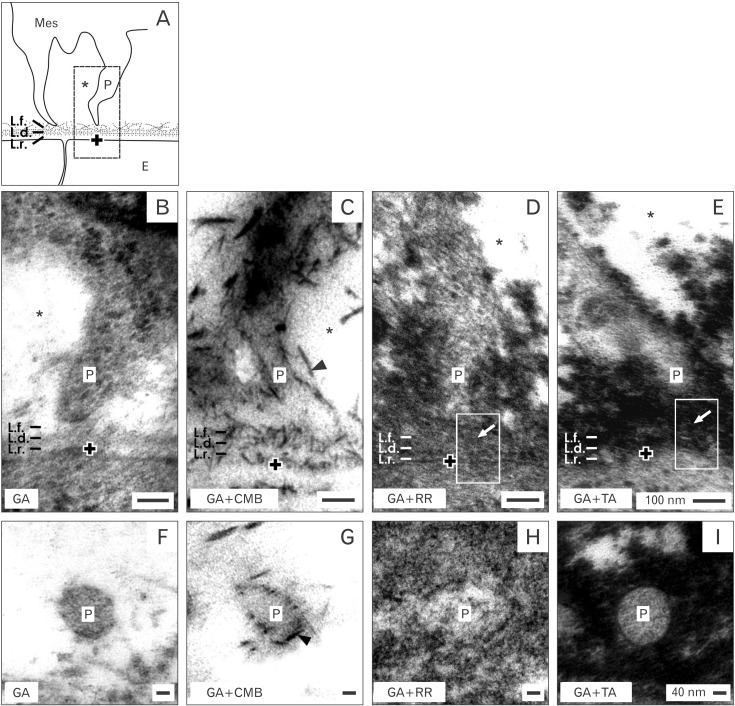
Fig. 5
(A-M) Analysis of the contact between the end of a mesenchymal cell projection and an epithelial cell within the stem/progenitor cell niche of neonatal rabbit kidney by transmission electron microscopy (B-I) and schematic illustration (J-M). Mes, mesenchymal cell; E, epithelial cell; P, projection. Specimens were fixed by traditional glutaraldehyde (GA) solution (B, F) and GA solution including cupromeronic blue (CMB) (C, G), ruthenium red (RR) (D, H), or tannic acid (TA) (E, I). In a vertical perspective it is recognized that the end of a mesenchymal cell projection is crossing the interface (asterisk) to have a special contact on the basal lamina of epithelial cells. The distance between the end of a projection and the plasma membrane of an epithelial cell is in average 167 nm. (B) Fixation in GA solution shows that the end of a projection has a contact on the basal lamina via tiny microfibers. (C) Fixation in GA solution including CMB illustrates that a cell projection is mounted in a cylinder of proteoglycan braces (arrowhead). (D) Fixation in GA solution including RR shows that a projection is integrated in a sleeve of extracellular matrix. (E) Fixation in GA solution including TA depicts that a projection is enveloped in labeled extracellular matrix. (F-I, J-M) In the center of a projection tunneling nanotubes (arrows) are seen connecting mesenchymal with epithelial cells. The basal lamina at the tip of a collecting duct ampulla consists of a lamina rara (L.r.), lamina densa (L.d.) and lamina fibroreticularis (L.f.). The basal plasma membrane of enclosed epithelial cell is marked by a cross (+).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download