Abstract
Vascular anomalies are frequently encountered in abdomen. But they are usually asymptomatic and diagnosed accidently during angiography or surgery leading into severe complications. Thus knowledge of angioarchitecture in abdomen, whether normal or variant, is considered prerequisite for successful, uncomplicated surgeries and interventional radiology. This case report describes one of such varying branching pattern of celiac trunk and superior mesenteric artery. During routine abdominal dissection, gastroduodenal artery was seen arising from celiac trunk along with its usual three branches. Common hepatic artery continued as left hepatic artery after giving rise the right gastric artery and a tortuous replaced right hepatic artery arose from superior mesenteric artery. An unusually long cystic artery arose from left hepatic artery and gave rise to 2-3 small anastomotic branches towards hepatic flexor of colon, in addition to its normal gallbladder supply. Awareness of such variations would certainly be helpful in upper abdominal surgeries.
Vascular anomalies are frequently encountered in abdomen. But they are usually asymptomatic and diagnosed accidently during diagnostic angiography or surgery/dissection [1]. Despite refinement in various abdominal surgical techniques, vascular complications still account for considerable morbidity and mortality. A detailed knowledge of the abdominal angioarchitecture, whether normal or variant, is thus considered a prerequisite for successful, uncom plicated abdominal surgeries and interventional radiological procedures [1, 2, 3].
Vitelline arteries, a number of paired vessels supplying the yolk sac, gradually fuse and form the arteries located in the dorsal mesentery of the developing gut. In the adult, they are represented by celiac trunk (CT), superior mesenteric artery (SMA), and inferior mesenteric artery (IMA) supplying the derivatives of fore-gut, mid-gut and hind-gut respectively. CT is first ventral branch of abdominal aorta, arising just below the aortic hiatus at the level of T12-L1 vertebrae and divides into left gastric artery (LGA), common hepatic artery (CHA), and splenic artery (SA) after a short course. SMA is the 2nd ventral branch of abdominal aorta, arising approximately 1 cm below the origin of CT at the level of L1 vertebra and gives rise inferior pancreaticoduodenal, jejunal, ileal, ileocolic, right colic and middle colic arteries in its normal course [4]. Anatomic variations of these visceral arteries are well known in medical literature; owing to multiple developmental pro cesses including persistence, incomplete regression or disappearance of part of primitive vitelline arteries [4] and these variations in abdominal angioarchitecture may puzzle the surgeons and vascular radiologists dealing with intra-abdominal diseases [1, 2, 3].
One of such case involving a rarely reported combination of varying branching pattern of CT and SMA is reported here.
During routine dissection in subhepatic region of abdomen of a well embalmed 70-year-old male cadaver in the Department of Anatomy, M. L. N. Medical College, Allahabad, India, on 15 May 2012, following variations in the branching pattern of CT and SMA were encountered.
The CT was seen arising from left lateral aspect of the abdominal aorta at the level between T12-L1 vertebrae instead of its usual ventral origin. After a short course of 7 mm the CT was observed to give rise an additional branch i.e., gastroduodenal artery (GDA) (diameter, 5 mm) in addition to its usual three branches viz. LGA (diameter, 4 mm), SA (diameter, 8 mm), and CHA (diameter, 7 mm) (Fig. 1). SA followed an unusual straight course towards left and was not tortuous as it should be. CHA gave off right gastric artery (RGA) after a course of 12 mm from its origin and continued as left hepatic artery (LHA). LHA ascended towards the liver in the right free margin of lesser omentum, anterior to portal vein and left to the common bile duct; and gave off an unusually long cystic artery (CA) (diameter, 4 mm) before entering into liver parenchyma at the porta hepatis. SMA arose from the ventral surface of abdominal aorta at the level of L1 vertebra, 22 mm distal to the origin of CT; and descended behind the neck of pancreas, where it gave off a "replaced" right hepatic artery (RHA) (diameter, 6 mm) for the supply of right lobe of liver. No right sided hepatic arterial system was identified from the CHA. This replaced RHA exhibited a long and tortuous course towards the liver and was crossed in front by CA before its entrance into the porta hepatis. The CA was running laterally after its origin from LHA, in front of replaced RHA and gave off 2-3 small anastomotic branches running down towards hepatic flexor of colon, in addition to its usual superficial and deep branches to the gallbladder (Figs. 1, 2).
No other anomalous blood vessel was identified. Diameter of all arteries was measured with the help of sliding vernier caliper and scale.
In recent times, the trend in surgical branches is to move towards minimal invasive surgery to decrease morbidity. Despite refinement in surgical techniques especially in hepatic region like laproscopic cholecystectomy or liver trans plant surgeries, vascular complications still account for considerable morbidity and mortality [2]. Conventional text book descriptions of the regional blood supply did not seem adequate in laparoscopic view till recent past and this was the time when anatomy of hepatic region was revisited and hepatic angioarchitecture was explored. Anatomical variations of the CT and SMA were observed frequently. According to Tandler [5], the anatomical variations of ventral branches of abdominal aorta are due to developmental changes in the ventral segmental (vitelline or splanchnic) arteries supplying yolk sac, allantois and chorion. During embryological period, there are longitudinal anastomoses connecting primitive segmental arteries in abdominal region. There is regression of all segmental arteries as development proceeds except for three, which remain as precursors of three major mesenteric vessels, and the longitudinal anastomotic vessels persisted in parts. The 10th and 13th and 21st or 22nd segmental arteries give rise to the CT, SMA, and IMA, respectively. Persistence, incomplete regression or disappearance of parts of these primitive ventral segmental arteries and their anastomotic vessels could give rise to numerous variations of branching pattern of CT, SMA, and IMA [4, 6].
Also the extrahepatic biliary system, developed from an intestinal diverticulum, carries a rich supply of vessels form aorta, CT and SMA. Later, most of these vessels are absorbed leaving the mature vascular system in place. Again as the pattern of absorption is highly variable, it is not unusual for CA and its branches to be derived from any other artery in the vicinity [6, 10]. Because of such variations of branching patterns of CT and SMA, due to developmental changes of the primitive ventral segmental arteries, liver may receives its blood supply directly or indirectly from CT, SMA, or even aorta [4].
Michels [7] classified the basic anatomical variations of hepatic arterial supply in 10 groups in his classic autopsy series of 200 dissections, published in 1955, and reported the variant patterns in 45% of cases which were designated as "accessory" or "replaced" arteries. Subsequently a number of studies classified these variations in different categories considering Michels' classifications as the benchmark. Hiatt et al. [8] reviewed the records of 1,000 patients who underwent liver harvesting for orthotopic transplantation between 1984 and 1993, and classified the hepatic arterial supply into six groups. They reported normal arterial anatomy in 75.7% patients against 55% in Michels' classification [8]. Mehta et al. [6] reported quadrifurcated hepatic artery proper (HAP) in conjunction with double RGA communicating distally forming an arterial loop. Baliyan et al. [9] reported a case with trifurcated HAP with unusual accessory LHA which did not supplied liver parenchyma despite traversed the fissure for ligamentum venosum. Several such reports are emerging with unique divergence in the hepatic arterial branching patterns which could not entirely addressed by conventional groupings. Present case also corresponds partly to the third type of both the classifications developed by Michels [7] and Hiatt et al. [8] (Table 1).
The CA usually (70-80%) arises within the Calot's triangle (bounded by cystic duct, common hepatic duct and inferior border of liver), from the right side of RHA and usually single. It also presents an unusually high degree of surgically important variability not only in its origin or number but also in its course to gallbladder [9]. Anson [10] in his landmark publication "Anatomical consideration in surgery of the gallbladder" described the origin of CA from following sources: RHA (63.9%), hepatic trunk (26.9%), LHA (5.5%) (described in present case) (Fig. 1), GDA (2.6%), superior pancreatico-duodenal artery (0.3%), RGA (0.1%), CT (0.3%), and SMA (0.8%).
Most of the authors reported and discussed anatomical varia tions of CT/SMA and CA separately, but during minimal invasive surgeries in subhepatic region, a detailed knowledge of all possible combinations of vascular variations is necessary to avoid vascular complications. Our case describes a combination of variations of branching pattern of CT, SMA as well as CA. "Multiplication rule" states that the pro bability of two or more statistically independent events occurring together is equal to the product of their individual probability. If the origin of RHA from SMA (11%, Michels) and CA from LHA (5.5%, Anson) would be considered statistically independent then the described combination of variations would have been found with a probability P=0.00605. Awareness of such a rare variant angioarchitecture with their embryological basis would certainly be helpful in upper abdominal surgeries especially laproscopic and liver transplant surgeries; and would enable interventional radiologists in planning and executing safe and successful procedures.
Figures and Tables
 | Fig. 1Photograph showing multiple anomalies of celiac trunk (CT) and superior mesenteric artery (SMA) and their branches with stomach and part of common hepatic artery (CHA)/left hepatic artery (LHA) removed (represented by a dotted line). CA, cystic artery; GB, gallbladder; GDA, gastroduodenal artery; LGA, left gastric artery; RHA, replaced right hepatic artery; SA, splenic artery. |
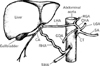 | Fig. 2Schematic representation of Fig. 1 showing multiple variations in arterial anatomy as observed in present case. CA, cystic artery; CHA, common hepatic artery; CT, celiac trunk; GDA, gastroduodenal artery; LGA, left gastric artery; LHA, left hepatic artery; RGA, right gastric artery; RHA, replaced right hepatic artery; SA, splenic artery; SMA, superior mesenteric artery. |
Acknowledgements
The authors would like to express sincere gratitude to their respected teacher Prof Dr A. K. Singh, Head of the Department of Anatomy, M. L. N. Medical College, Allahabad, India for his permission and able guidance to perform this study.
References
1. Raikos A, Paraskevas GK, Natsis K, Tzikas A, Njau SN. Multiple variations in the branching pattern of the abdominal aorta. Rom J Morphol Embryol. 2010; 51:585–587.
2. Gielecki J, Zurada A, Sonpal N, Jabłońska B. The clinical relevance of coeliac trunk variations. Folia Morphol (Warsz). 2005; 64:123–129.
3. Loukas M, Shah R, Tubbs S, Merbs W. Multiple variations of the hepatobiliary vasculature including a splenomesenteric trunk. Singapore Med J. 2010; 51:e6–e8.
4. Kalthur SG, Sarda R, Bankar M. Multiple vascular variations of abdominal vessels in a male cadaver: embryological perspective and clinical importance. J Morphol Sci. 2011; 28:152–156.
5. Tandler J. Uber die Varietaten der Arteria coeliaca und deren Entwickelung. Anat Hefte. 1904; 25:473–500.
6. Mehta V, Dave V, Suri RK, Rath G. Quadrifurcation of the hepatic artery proper in conjunction with double right gastric arteries. Singapore Med J. 2012; 53:e211–e213.
7. Michels NA. Newer anatomy of the liver and its variant blood supply and collateral circulation. Am J Surg. 1966; 112:337–347.
8. Hiatt JR, Gabbay J, Busuttil RW. Surgical anatomy of the hepatic arteries in 1000 cases. Ann Surg. 1994; 220:50–52.
9. Baliyan R, Mehta V, Suri RK, Arora J, Rath G. Anatomic divergence in the hepatic arterial branching pattern: clinical viewpoint value to hepatologists. Br J Med Health Sci. 2012; 1:39–45.
10. Anson BH. The aortic arch and its branches. In : Luisada AA, editor. Cardiology. Vol. 1. New York: McGraw-Hill;1963. p. 119.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download