Abstract
CD10, a marker of immature B lymphocytes, is expressed in the developing epithelium of mammary glands, hair follicles, and renal tubules of human fetuses. To assess mesenchymal and stromal expression of CD10, we performed immunohistochemical assays in whole body sections from eight fetuses of gestational ages 15-20 weeks. In addition to expression in urinary tract and intestinal epithelium, CD10 was strongly expressed at both gestational ages in fibrous tissues surrounding the airways from the larynx to lung alveoli, in the periosteum and ossification center, and in the glans of external genitalia. CD10 was not expressed, however, in other cavernous tissues. These findings suggest that mesenchymal, in addition to epithelial cells at specific sites, are likely to express CD10. The glomeruli, alveoli, and glans are all end products of budding or outgrowth processes in the epithelium or skin. However, in contrast to the CD34 marker of stromal stem cells, CD10 was not expressed in vascular progenitor cells and in differentiated vascular endothelium. The alternating pattern of CD10 and CD34 expression suggests that these factors play different roles in cellular differentiation and proliferation of the kidneys, airway and external genitalia.
CD10, expressed by acute lymphoblastic leukemia cells, is a marker of immature B lymphocytes (e.g., Barcena et al. [1]) which are defined as CD10-positive, IgM-negative cells [2]. CD10-positive lymphocytes constitute 0.3% of peripheral blood and 0.5% of cord blood cells [3], and CD10 was recently shown to be a stem cell marker in hair follicles [4] and mammary glands [5]. Stage-dependent changes in their reactive site have been observed in the developing renal tubular system [6], providing further evidence that all CD10-positive cells outside the immune and hematopoietic systems are of epithelial, not mesenchymal or stromal origin.
CD34 has long been regarded as a stem cell marker. CD34-positive cells have been classified as hematopoietic (HLA-DR positive), mesenchymal, and stromal (HLA-DR negative) progenitor cells [7]. Our group previously reported that CD34-positive fibrous tissues are present both in subcutaneous tissue and connective tissues located in and around striated muscles, tendons and ligaments in human mid-term fetuses [8, 9]. Some CD34-positive connective tissues seemed to overlap with, or are proximal to CD10-positive sites, although the latter has not been fully examined. We also found CD10-positive non-blood cells distributed in CD34-positive mesenchymal tissues in midterm fetuses (15 weeks) during a recent study of lymphocyte differentiation in fetal nodes (submitted to Anatomy and Cell Biology). Thus, we needed to compare the finding with the later stages.
These results suggest that similar to CD34, CD10 may be expressed not only in epithelial, but also in mesenchymal and/or stromal tissues located along and around the epithelium in human fetuses. The purpose of this study was to comprehensively analyze CD10 expression sites, and compare CD10 and CD34 expression patterns in the human mid-term fetus.
The study was performed in accordance with the Ethical Principles for Medical Research Involving Human Subjects, outlined in the Declaration of Helsinki 1995 (as revised in Edinburgh 2000). We analyzed paraffin-embedded samples of eight mid-term fetuses: five fetuses of gestational age (GA) 15 weeks (102-120 mm crown-rump length [CRL]) and three fetuses of GA 20 weeks (155-170 mm CRL). With the agreement of the families concerned, these specimens were donated to the Department of Surgery, Chonbuk National University in Korea, and their use for research was approved by the university ethics committee (CUH 2013-03-007). In compliance with all university and hospital rules, authors other than those at Chonbuk University were not required to inform the corresponding committees in Japan about this research project. The fetuses had been obtained following induced pregnancy termination upon request. Following the termination, each woman was personally asked by an obstetrician about the possibility of donating the fetus for research; no attempt was made to encourage donations. Because of randomized specimen numbering, it was not possible to trace any of the families concerned.
Donated fetuses were fixed in 10% w/w neutral formalin solution for more than 1 month. Each was divided into regions encompassing the head and neck, the thorax, abdomen, pelvis and each of the four extremities. All sections were decalcified by incubating in 0.5-mol/l ethylenediaminetetraacetic acid at 4℃ (pH 7.5, Decalcifying Solution B, Wako, Tokyo, Japan) for 1-3 days, depending on the size of the body part. At 50-µm intervals, specimens of the head and neck, thorax, abdomen and the pelvis were processed for sagittal or horizontal sectioning, with each section 5 µm in thickness.
Most sections were stained with hematoxylin and eosin, with others used for immunohistochemical assays. The primary antibodies used were 1) mouse monoclonal anti-human CD10 (1:100, NCL-CD10-270, Novocastra, Newcastle upon Tyne, UK) and 2) mouse monoclonal anti-human CD34 class II (1:100, Dako M7165, Dako, Glostrup, Denmark). The secondary antibody (Dako Chem Mate Envison Kit) had been labeled with horseradish peroxidase (HRP), and antigen-antibody reactions were detected via the HRP-catalyzed reaction with diaminobenzidine. All samples were subsequently counterstained with hematoxylin.
CD10-positive immature B lymphocytes were observed in the thymus cortices of fetuses at 15 weeks GA. At 20 weeks GA, B cells were also present in the periportal fibrous tissue of the liver, the periarterial sheath of the spleen and associated lymph nodes (manuscript in preparation). CD10 was also strongly expressed in the kidneys, airways, and external genitalia. This suggests that CD10 is expressed in specific epithelium and fibrous tissue, whereas CD34 is widely expressed in developing vessels and musculocutaneous fibrous tissues. CD34 expression was specifically different in the intrahepatic vascular system; branches of the portal vein and hepatic artery were positive for CD34, whereas the thin tributaries of the hepatic veins were negative, both at 15 and 20 weeks. CD34-positive cells formed rosette-like structures, possibly corresponding to primitive bile ducts (Fig. 1F). Even at 20 weeks, however, the developing sinusoid in the liver was negative for CD34.
Epithelial expression of CD10 was restricted to specific sites. In the kidneys, the glomerulus and proximal tubules were positive at 15 weeks GA, which extended to Henle's loop and parts of the distal tubule and collecting duct at 20 weeks (Fig. 1A-D). At both stages, however, newly developing glomeruli present in the most superficial layer of the renal cortex were negative. In developed glomeruli, the tubular and capsular components were positive, but the vascular and mesangial components were negative. CD34 was not expressed in the tubular system of the kidneys but was present in all vessels and some fibrous tissues, including the renal capsule (Fig. 1B, D). In the alimentary canal epithelium, CD10 was expressed at the luminal or apical surfaces of the villi of the duodenum, jejunum and ileum (Fig. 2A, B), as well as in the basal layer of the oral and epiglottic mucosa (Fig. 3A). The internal enamel epithelium of the tooth buds was also positive. However, in the salivary glands (Fig. 3A) and pancreas (Fig. 2C), the epithelium of the duct and acinus was negative for CD10. Moreover, the epithelium of the pharynx, esophagus and stomach (Figs. 2E, 4C), in addition to the airways (Fig. 4), was negative for CD10. The colon and rectum were negative for CD10, whereas the basal epithelial layers of the renal pelvis, ureter, bladder and urethra were CD10-positive (Fig. 5A, C). However, the colon and rectum contained a CD10-positive meconium-like substance in the lumen (Fig. 5A, C).
CD34 expression in the alimentary canal and urogenital tract was restricted to the vessels and the serosal and mesenteric fibrous tissues surrounding them. In the pancreas, however, fibrous and vascular tissues, in addition to the capsule of acinus, strongly expressed CD34 (Fig. 2D). Hair follicles of the skin were positive for CD10 at 20 weeks (Fig. 3E), whereas CD34 expression was stronger in the fibrous sheaths of the follicles compared to the surrounding subcutaneous tissue (Fig. 3F).
Assessment of the mesothelium showed CD10 expression in the pericardium and CD34 expression in the pericardium, pleura and peritoneum (Figs. 2, 4).
The most striking example of CD10 expression was observed in fibrous tissues, especially in the airways (Figs. 3, 4) and glans of the external genitalia (Fig. 6). In the airways, CD10 was expressed in fibrous tissues along and around the laryngeal, tracheal, and bronchial cartilage as well as around glandular phase lung alveoli. The cartilages themselves, however, were negative. In contrast, CD34 was expressed in all vascular endothelial tissues, including the very thin alveolar vessels in the lungs. The adventitia of thick vessels, especially of intrapulmonary arteries, was also strongly positive for CD34. These findings suggest that patterns of CD10 and CD34 expression in the lungs alternate, with the bronchi positive for CD10 and the arteries positive for CD34 (Fig. 4). Likewise, submucosal fibrous tissue in the larynx was positive for CD10 and negative for CD34. The glans of the external genitalia in both genders was strongly positive for CD10, whereas other cavernous tissues were CD10-negative. Thin vessels, as well as the tunica albuginea of cavernous tissues in external genitalia, strongly expressed CD34 (Fig. 6). Although the glans also contained CD34-positive thin vessels, this alternating expression pattern of CD10 and CD34 was evident at 20 weeks, with the dark-colored glans positive for CD10 and the pale-colored glans containing CD34-positive thin vessels (Fig. 6C, D).
At 15 weeks GA, fibrous tissues in and along the striated and cardiac muscles were strongly positive for CD34 but negative for CD10 (Figs. 3A, B, 7A, B). At 20 weeks, however, fibrous tissues in and around the striated muscles, in addition to the adventitia of thick arteries and veins, were weakly positive for CD10 (Figs. 4E, 7E). CD10 was also expressed along some cartilage and bone such as the craniovertebral junction and Meckel's and Reichert's cartilages (Fig. 3A). Spindle-shaped and round cells in the membranous (Fig. 3A, B) and endochondral (Fig. 7A, B) ossification centers were positive for both CD10 and CD34. The joint membranes, synovial tissues, and ligaments were strongly positive for CD34 (Fig. 7D), but weakly positive for CD10 (Fig. 7C). CD10-positive fibrous tissues were also seen around the vagina and bladder (Fig. 5A, C). No expression of CD10 was found in the brain and internal ear, except at the pial surface of the choroid plexus. In contrast, the brain and spinal cord contained abundant CD34-positive vessels, as we previously reported [10]. The tympanic membrane and external acoustic meatus were positive for CD10, as were the ciliary muscle, eyelid and putative lateral ligament. These findings are summarized in Table 1.
CD10 is a marker of developing epithelium, expressed in the epithelium of renal tubules and hair follicles but not in newly developing glomeruli at the most superficial layer of the renal cortex. Thus, rather than being an inducer or regulator, CD10 expression in the kidney may simply be the result of differentiation. In the small intestine, CD10 expression was restricted to the luminal surface of the villi. The colon and rectum contained a CD10-positive meconium-like substance in the lumen, suggesting that the small intestine did not express CD10 but secreted a CD10-like substance. In the urinary tract, CD10 expression was restricted to the basal layer of the epithelium, a pattern also observed for proteins such as podoplanin and D2-40 [11]. This suggests that CD10 may have a limited role in the epithelium. Conversely, CD10 seems to play a role in mesenchymal differentiation and proliferation. Furthermore, the epithelial expression of CD34 (a marker of stromal stem cells) was limited to rosette-like structures in liver paracaval tissue, originating from the ductal plate [12] and containing abundant numbers of stem cells [13]. Overall, CD10 and CD34 seem to play role(s) in different aspects of mesenchymal tissue differentiation.
To our knowledge, this is the first study to show that CD10 is expressed in fetal fibrous and mesenchymal tissues. In contrast to CD34 expression, which is widely distributed throughout muscle-associated and subcutaneous tissues, CD10 expression in mesenchymal tissue was restricted to specific sites including the glomeruli, airways, and glans of the external genitalia. These three structures are all end portions in the budding and/or outgrowth processes of the epithelium and surface ectoderm. Therefore, despite exceptions such as the salivary glands and pancreas, CD10 is likely to play a role in populations of mesenchymal cells responsible for epithelial-mesenchymal interactions during budding or outgrowth. The most distal airways with no cartilages were strongly positive for CD10, with CD10-positive fibrous tissues likely involved in the maintenance, development, and growth of tracheobronchial cartilages. Tracheobronchomalacia, a congenital or acquired condition characterized by the softening of airway cartilages, is likely to accompany abnormalities of the hair, glans, eyes, ears, and teeth such as Hallermann-Streiff syndrome [14, 15]. Further examinations of patients may help define a subtype of tracheobronchomalacia associated with functional CD10 defects.
We observed differences in the expression patterns of CD10 and CD34, particularly in the airways. This was especially notable in the lung and the glans of external genitalia. Almost all visceral interstitial tissues, such as fibrous tissues in the alimentary canal and urogenital tract, were negative for CD34. Unlike in the viscera, CD34 is expressed in striated muscle-associated connective tissue and subcutaneous tissue. Using CD34-positive cell lines from 12-week-old fetuses, site-dependent differences in E-selectin, intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 expression were observed following administration of interleukin-1 beta or tumor necrosis factor alpha [16]. Expression of these adhesion molecules by CD34-positive fibrous tissues in the developing muscle and associated structures may accelerate the formation of muscle fiber and tendon bundles. CD10 may also induce expression of these adhesion molecules, or even play a role in adhesion itself. However, the alternating expression patterns of CD10 and CD34 suggest that their roles in differentiation and proliferation are distinct.
Although we took great care, immunoreactivity of mesenchymal tissues was similar to background staining. We did not compare results in a single fetus, but used multiple fetuses to illustrate many sites in the body. Evaluating differences in immunoreactivity between stages may be more difficult owing to individual variations. Some specimens may display a high degree of non-specific reactivity, whereas other specimens may show little or none. Therefore, it may be inappropriate to discuss whether CD10 or CD34 expression was relatively strong or weak.
Figures and Tables
Fig. 1
Immunohistochemistry of CD10 (A, C, E) and CD34 (B, D, F) in the kidney and liver. Panels (A) and (B) (near sections) show a kidney of gestational age (GA) 15 weeks, whereas panels (C) and (D) (near sections) show kidneys at GA 20 weeks. Inserts between panels (A) and (C) and between panels (B) and (D) (scale bars=0.1 mm) show more highly magnified views of a glomerulus in panels (C) and (D). Panels (E) and (F) (near sections) show the liver parenchyma around a portal vein branch at 15 weeks. The superficial layer of the renal cortex (stars in panels A and C) was negative for CD10. Renal capsules were positive for CD34 (arrowheads in panels B and D). CD10-positive immature B lymphocytes were present in a portal vein branch (arrows in panel E), whereas CD34-positive cells formed rosette-like structures (arrows in panel F). Panels (A-D) (E and F) were prepared at the same magnification. Scale bars in panels (A) and (E)=1 mm (A-D), 0.1 mm (E, F).
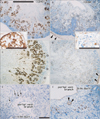
Fig. 2
Immunohistochemical expression of CD10 (A, C, E) and CD34 (B, D, F) in the intestines, pancreas, and stomach. Panels (A) and (B) (near sections) show the ileum at gestational age (GA) 15 weeks, whereas panels (C) and (D) (near sections) show the duodenum and pancreas at GA 20 weeks. Panels (E) and (F) (near sections) show the stomach at GA 15 weeks. CD10 was positive at the luminal surface of the intestinal villi (A, C), but not in the stomach (E). CD10-positive immature B lymphocytes are shown in panel (C). (B, D) The mesentery and pancreas showed strong expression of CD34. SMA, superior mesenteric artery. All panels were prepared at the same magnification. Scale bar in panel (A)=1 mm (A-F).
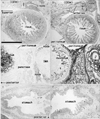
Fig. 3
Immunohistochemical expression of CD10 (A, C, E) and CD34 (B, D, F) in the mandible, larynx, and hair. Panels (A) and (B) (near sections) show the mandible containing Meckel's cartilage from a fetus of gestational age (GA) 15 weeks, and panels (C) and (D) (near sections) show the larynx of the same fetus. Panels (E) and (F) (near sections) show a hair follicle in the anterior thoracic skin at GA 20 weeks. CD10-positive fibrous tissues were present in the larynx (C) and along Meckel's cartilage (A), whereas CD34-positive fibrous tissues were abundant in striated muscles (stars in panels B and D), the sublingual gland (SLG in panel B) and subcutaneous tissue (D, F). PH, epithelium of the posterior wall of the pharynx. Panels (A-D) (or E and F) were prepared at the same magnification. Scale bars in panels (A) and (E)=1 mm (A-D), 0.1 mm (E, F).
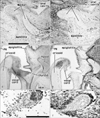
Fig. 4
Immunohistochemical expression of CD10 (A, C, E) and CD34 (B, D, F) in the lungs and mediastinum. Panels (A) and (B) (near sections) show the right pulmonary hilus at gestational age (GA) 15 weeks, while panels (C) and (D) (near sections) show branching of the subsegmental bronchi and arteries in segment VI of the right lung at GA 20 weeks. The bronchi are surrounded by CD10-positive fibrous tissues, whereas the arteries are surrounded by CD34-positive tissues. The connective tissues of the trachea also express CD10 strongly. The endothelium of the aorta and pulmonary arteries express CD34 (arrowheads in panels B, D, and F). Panels (E) and (F) (near sections) show mediastinal structures at GA 15 weeks. All panels were prepared at the same magnification. Scale bar in panel (A)=1 mm (A-F).
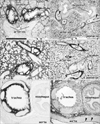
Fig. 5
Immunohistochemical expression of CD10 (A, C) and CD34 (B, D) in the bladder, vagina, and rectum. Panels (A) and (B) (near sections) show the bladder, vagina, and rectum at gestational age (GA) 15 weeks, while panels (C) and (D) (adjacent sections) show these three structures at GA 20 weeks. CD10 expression was observed in the bladder epithelium and the adventitia of the vagina, whereas the thin vessels and retropubic fibrous tissue were positive for CD34 (stars in panel B). A CD10-positive meconium-like substance was observed in the rectum (stars in panels A and C). Asterisks in panel (D) indicate an artifactual cleft generated during the histological procedure. All panels were prepared at the same magnification. Scale bar in panel (A)=1 mm (A-D).
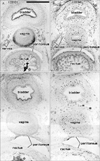
Fig. 6
Immunohistochemical expression of CD10 (A, C) and CD34 (B, D) in the clitoris. Panels (A) and (B) (near sections) display the clitoris and labium at gestational age (GA) 15 weeks, while panels (C) and (D) (adjacent sections) show the clitoris at GA 20 weeks. CD10-positive fibrous tissues were present in the subcutaneous tissue and glans of the clitoris, whereas CD34 was positive in thin vessels and in some CD10-positive fibrous tissues. The tunica albuginea of cavernous tissues was positive for CD34 (stars in panel D), but negative for CD10 (stars in panel C). All panels were prepared at the same magnification. Scale bar in panel (A)=1 mm (A-D).
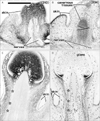
Fig. 7
Immunohistochemical expression of CD10 (A, C, E) and CD34 (B, D, F) in bones, joints and muscles. Panels (A) and (B) (near sections) show the ilium at gestational age (GA) 15 weeks, panels (C) and (D) (adjacent sections) show the hip joint at GA 20 weeks, and panels (E) and (F) (adjacent sections) show the gluteal muscles and the skin covering them at GA 20 weeks. The endochondral ossification center (stars in panels A and B) as well as the synovial tissue (panels C and D) were positive for both CD10 and CD34. Striated muscle fibers were separated by abundant sheaths strongly positive for CD34 (F) and weakly positive for CD10 (E). All panels were prepared at the same magnification. Scale bar in panel (A)=1 mm (A-F).
Acknowledgements
This study was supported by a grant (0620220-1) from the National R & D Program for Cancer Control, Ministry of Health & Welfare, Republic of Korea.
References
1. Bárcena A, Muench MO, Galy AH, Cupp J, Roncarolo MG, Phillips JH, Spits H. Phenotypic and functional analysis of T-cell precursors in the human fetal liver and thymus: CD7 expression in the early stages of T- and myeloid-cell development. Blood. 1993; 82:3401–3414.
2. Paramithiotis E, Cooper MD. Memory B lymphocytes migrate to bone marrow in humans. Proc Natl Acad Sci U S A. 1997; 94:208–212.
3. D'Arena G, Musto P, Cascavilla N, Di Giorgio G, Fusilli S, Zendoli F, Carotenuto M. Flow cytometric characterization of human umbilical cord blood lymphocytes: immunophenotypic features. Haematologica. 1998; 83:197–203.
4. Poblet E, Jiménez F. CD10 and CD34 in fetal and adult human hair follicles: dynamic changes in their immunohistochemical expression during embryogenesis and hair cycling. Br J Dermatol. 2008; 159:646–652.
5. Bachelard-Cascales E, Chapellier M, Delay E, Pochon G, Voeltzel T, Puisieux A, Caron de Fromentel C, Maguer-Satta V. The CD10 enzyme is a key player to identify and regulate human mammary stem cells. Stem Cells. 2010; 28:1081–1088.
6. Faa G, Gerosa C, Fanni D, Nemolato S, Marinelli V, Locci A, Senes G, Mais V, Van Eyken P, Iacovidou N, Monga G, Fanos V. CD10 in the developing human kidney: immunoreactivity and possible role in renal embryogenesis. J Matern Fetal Neonatal Med. 2012; 25:904–911.
7. Waller EK, Huang S, Terstappen L. Changes in the growth properties of CD34+, CD38- bone marrow progenitors during human fetal development. Blood. 1995; 86:710–718.
8. Abe S, Suzuki M, Cho KH, Murakami G, Cho BH, Ide Y. CD34-positive developing vessels and other structures in human fetuses: an immunohistochemical study. Surg Radiol Anat. 2011; 33:919–927.
9. Katori Y, Kiyokawa H, Kawase T, Murakami G, Cho BH. CD34-positive primitive vessels and other structures in human fetuses: an immunohistochemical study. Acta Otolaryngol. 2011; 131:1086–1090.
10. Chang H, Cho KH, Hayashi S, Kim JH, Abe H, Rodriguez-Vazquez JF, Murakami G. Site- and stage-dependent differences in vascular density of the human fetal brain. Childs Nerv Syst. 2014; 30:399–409.
11. Yajin S, Murakami G, Takeuchi H, Hasegawa T, Kitano H. The normal configuration and interindividual differences in intramural lymphatic vessels of the esophagus. J Thorac Cardiovasc Surg. 2009; 137:1406–1414.
12. Moon WS, Cho BH, Hayashi S, Kim JH, Murakami G, Fukuzawa Y, Nakano T. Cytokeratin-positive hepatocytes in the hilar region: an immunohistochemical study using livers from fetuses and elderly individuals. Ann Anat. 2011; 193:224–230.
13. Gerlach JC, Over P, Turner ME, Thompson RL, Foka HG, Chen WC, Péault B, Gridelli B, Schmelzer E. Perivascular mesenchymal progenitors in human fetal and adult liver. Stem Cells Dev. 2012; 21:3258–3269.
14. Kano Y, Sakurai H, Shidara J, Toida S, Yasuda H. Histopathological and immunohistochemical studies of acquired tracheobronchomalacia: an autopsy case report. ORL J Otorhinolaryngol Relat Spec. 1996; 58:288–294.
15. Carden KA, Boiselle PM, Waltz DA, Ernst A. Tracheomalacia and tracheobronchomalacia in children and adults: an in-depth review. Chest. 2005; 127:984–1005.
16. Invernici G, Ponti D, Corsini E, Cristini S, Frigerio S, Colombo A, Parati E, Alessandri G. Human microvascular endothelial cells from different fetal organs demonstrate organ-specific CAM expression. Exp Cell Res. 2005; 308:273–282.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download