Abstract
A term "mesoesophagus" has been often used by surgeons, but the morphology was not described well. To better understand the structures attaching the human abdominal and lower thoracic esophagus to the body wall, we examined serial or semiserial sections from 10 embryos and 9 fetuses. The esophagus was initially embedded in a large posterior mesenchymal tissue, which included the vertebral column and aorta. Below the tracheal bifurcation at the fifth week, the esophagus formed a mesentery-like structure, which we call the "mesoesophagus," that was sculpted by the enlarging lungs and pleural cavity. The pneumatoenteric recess of the pleuroperitoneal canal was observed in the lowest part of the mesoesophagus. At the seventh week, the mesoesophagus was divided into the upper long and lower short parts by the diaphragm. Near the esophageal hiatus, the pleural cavity provided 1 or 2 recesses in the upper side, while the fetal adrenal gland in the left side was attached to the lower side of the mesoesophagus. At the 10th and 18th week, the mesoesophagus remained along the lower thoracic esophagus, but the abdominal esophagus attached to the diaphragm instead of to the left adrenal. The mesoesophagus did not contain any blood vessels from the aorta and to the azygos vein. The posterior attachment of the abdominal esophagus seemed to develop to the major part of the phrenoesophageal membrane with modification from the increased mass of the left fetal adrenal. After postnatal degeneration of the fetal adrenal, the abdominal esophagus might again obtain a mesentery. Consequently, the mesoesophagus seemed to correspond to a small area containing the pulmonary ligament and aorta in adults.
Hamilton and Mossman [1] reported that a true mesentery is absent from the fetal esophagus. In this context, the "true mesentery" seemed to indicate a serosal or fascial structure for vascular supply, such as the mid-gut mesentery containing the superior mesenteric artery and vein. However, a study of the pleuroperitoneal canal showed that the lungs and esophagus together contain a common mesentery-like structure that connects to the aorta at the 5th-6th week [2]. This mesentery-like structure is present at the 18th week and most likely contains lymphatic vessels but no blood vessels [3]. The mesentery-like structure of the esophagus should develop along with the lungs and pleural cavity, but little information is currently available.
Bilateral recesses of the celomic cavity (i.e., the pneumatoenteric recesses) are observed along and near the future gastro-esophageal junction, suggesting that these recesses are a primitive form of the upper part of the omental bursa or lesser sac [4]. Morphologic examination indicated that the left pneumatoenteric recess protrudes into and ends at the adrenal and soon disappears, whereas the right recess evolved into the superior recess of the omental bursa [2]. However, since most of these observations were based on horizontal sections, the left pneumatoenteric recess may not be distinguishable from the usual posterior recess of the peritoneal cavity extending between the adrenal and dorsal mesogastrium [5, 6]. Little information is therefore available about the topographical relationship between the pneumatoenteric recess and the mesentery-like structure of the esophagus and lungs. In adults, the posterior aspect of the abdominal esophagus is attached to the left crus of the diaphragm in front of the tenth and eleventh thoracic vertebrae [7]. It is not clear, however, whether this posterior attachment to the diaphragm corresponds to the lowest part of the fetal mesentery-like structure after deletion of the left peritoneal recess. This study therefore assessed the fetal development of the posterior attachment of the esophagus, especially of the mesentery-like structure, using both horizontal and sagittal sections.
The study was performed in accordance with the provisions of the Declaration of Helsinki 1995 (as revised in Edinburgh 2000). Five micron thick sagittal and horizontal paraffin sections stained with hematoxylin and eosin had been obtained from ten embryos and nine fetuses, including from four embryos of crown-rump length (CRL) 9-13 mm (approximately 5 weeks), six embryos of CRL 20-24 mm (7 weeks), seven fetuses of CRL 49-56 mm (10 weeks) and two fetuses of CRL 150-160 mm (18 weeks). Sections from the 5, 7, and 10 week specimens were serial, while those from the 18 week specimens were prepared at 50 mm intervals.
The nine 10- and 18-week-old fetal specimens had been donated by their respective families to the Department of Anatomy of Chonbuk National University, Korea, and their use for research was approved by the university ethics committee (No. CUH 2013-03-007). Each fetus had been obtained via induced abortion, after which the mother had been orally informed by an obstetrician (at a single hospital) about the possibility of donation for research; no attempt was made to actively encourage the donation. After the mother subsequently agreed, the fetus was assigned a specimen number and stored in 10% w/w formalin solution for more than 3 months. Because specimen numbers were randomized, there was no possibility of contacting the family at a later date.
The ten embryonic specimens were part of the large collection kept at the Embryology Institute, Universitad Complutense, Madrid, and were products of miscarriages and ectopic pregnancies managed at the Department of Obstetrics at the university. The study protocol was approved by our university ethics committee (No. B08/374). Our previous studies [2, 3, 5, 8] used thoracic and abdominal regions of the same specimens, with no anomalies observed.
The pharynx and upper esophagus of each of the four 5-week embryos were more than 2 times longer than the lower esophagus below the tracheal bifurcation, a finding likely due to the underdevelopment of the lungs (Fig. 1). Despite the very small size of the pleural cavity and the absence of a diaphragm, the position of the future esophageal hiatus could be estimated by the upper end of the superior recess of the omental bursa or lesser sac (Fig. 1A). Another, posterior peritoneal recess always extended upward behind the esophagus to the level of the developing left atrium (Fig. 1B, C). The stomach and left lung were separated by a narrow recess of the celomic or peritoneal cavity (Fig. 1C). The primitive superior recess of the omental bursa, corresponding to the right pneumatoenteric recess, was observed in horizontal sections, while one of the posterior peritoneal recesses was identified as the left pneumatoenteric recess (Fig. 2). Moreover, depending on the sizes of the lungs and pleural cavity below the tracheal bifurcation, a mesentery-like structure, which we have named the "mesoesophagus," became evident between the aorta and esophagus. The mesoesophagus was also connected to both lungs: a connection corresponding to the future pulmonary ligament. Before the formation of a venous communication between the liver and the fetal adrenal in the right side (i.e., the inferior vena cava [5]), these organs were tightly attached to each other and were located immediately to the right of the mesoesophagus. The apex of the lung was very low and appeared near the level of the tracheal bifurcation (Fig. 2A). The mesoesophagus, however, did not contain any arteries and veins.
At the 7th week (6 embryos), the pharynx and upper esophagus were still longer than the lower esophagus below the tracheal bifurcation. The mesoesophagus was consistently interrupted by the diaphragm, but the abdominal esophagus was too dilated to identify the final gastro-esophageal junction (Figs. 3, 4). Striated muscle fibers of the diaphragm were concentrated along and around the esophagus. Above the diaphragm, the esophagus was surrounded by bilateral recesses of the pleural cavity protruding into the mesoesophagus (Fig. 3C-F). Similarly, below the diaphragm, a posterior recess of the peritoneal cavity extended upward between the abdominal esophagus and the fetal adrenal gland in the left side or a large mass of the celiac ganglia, while the other, more anterior recess was continuous with the omental bursa that had expanded because it contained the liver caudate lobe (Fig. 4D, E). Despite the peritoneal recess, the left adrenal was usually (5 of the 6 specimens) attached to and protruded into the mesoesophagus along the abdominal esophagus. The pneumatoenteric recess was not identified in the mesoesophagus. Below the tracheal bifurcation, the mesoesophagus contained no arteries from the aorta or veins to the azygos vein. Because the heart was present in the anterior half of the thorax, the lungs did not reach the anterior thoracic wall.
At the 10th and 18th week (9 fetuses), the abdominal esophagus was consistently attached to the diaphragm on the left side (Figs. 5, 6). Striated muscle fibers of the diaphragm were seen along the esophagus as well as behind the adrenal. A fascia in the attachment site extended inferiorly and was continuous with the retropancreatic fascia between the pancreas body and left adrenal (Fig. 5B, C). Attachment between the esophagus and diaphragm on the right side was prevented by a large mass of the liver caudate lobe protruding into the omental bursa. In all specimens, the mesoesophagus could still be clearly identified (Fig. 6B, C) and the accompanying pulmonary ligament contained a recess of the pleural cavity. Similarly, in 3 of the 9 specimens, the peritoneal cavity provided a recess protruding into the lowest part of the mesoesophagus (Fig. 5D). The left adrenal and stomach were separated by one or more recesses of the peritoneal cavity (Figs. 5E, 6C-E). The distance between the tracheal bifurcation and the stomach was shorter than or equal to the length of the pharynx and upper esophagus. The lungs extended much higher than the level of the tracheal bifurcation.
These findings indicated that 1) the mesoesophagus was established at the 5th week and remained present at the 18th week; 2) pleural and peritoneal recesses protruded into the mesoesophagus at all stages examined; and 3) the abdominal esophagus and the left adrenal became attached at the 7th week but were again separated by a posterior peritoneal recess at the 10th week. The celomic, pleural and/or peritoneal recesses, those disturbed a mesentery-like configuration of the mesoesophagus, are schematically drawn in Fig. 7.
This study showed that the fetal esophagus contains a mesentery-like structure, which we have called the mesoesophagus. The rapid increase in the sizes of both lungs and pleural cavity sculpted the posterior mesenchymal tissue in front of the vertebral column to provide the narrow mesentery. Above the level of the tracheal bifurcation, however, there was no increasing lateral mass. After birth, the mesoesophagus likely becomes unclear or flat since the esophagus is pressed on the aorta by the increased lung volume. Because it was connected to both lungs, the mesoesophagus seemed to correspond to a small area that included the pulmonary ligament and aorta in adults. Thus, surgeons may overemphasize the supero-inferior extent of the mesoesophagus in adults because they consider it a surgical plane behind the esophagus [9].
We found that the pleural and peritoneal recesses protruding into or surrounding the mesoesophagus assumed various morphologies. A classical entity, the pneumatoenteric recesses, may correspond to one type of peritoneal recess. Our observations indicate that the left pneumatoenteric recess initially developed from one of the multiple peritoneal recesses extending between the stomach and left adrenal. When this recess was fused or obliterated, a fascia in the site remains continuous with the retropancreatic fusion fascia [10]. The right pneumatoenteric recess was reported to become the superior recess of the omental bursa or lesser sac [2, 4]. Therefore, the pneumatoenteric recess was likely not a specifically primitive or temporal structure but one of typical peritoneal structures along the abdominal esophagus.
The number of elastic fibers was found to be larger in the peritoneum than in the adjacent fascial structures [11, 12]. Because of the pleural and/or peritoneal remnants in the mesoesophagus, the esophageal hiatus seems to carry elastic fibers at the 10th week [13], much earlier than other tissues [14]. Surgeons have been interested in the morphology of the phrenoesophageal membrane (=the phrenicoesophageal ligament), a dome-like membrane surrounding the lower thoracic esophagus just above the esophageal hiatus (e.g., St Peter et al. [15]). The anterior part of the membrane is usually emphasized [16, 17], but the major part extends far inferiorly to provide the posterior wall of the hiatus [18]. The site of attachment of the fetal abdominal esophagus to the left-sided diaphragm likely becomes the major part of the phrenoesophageal membrane. Watanabe and Lister [13] assessed the initial morphology of the phrenoesophageal membrane at the 10th week, but we did not find any domelike fibrous structures in or above the fetal hiatus. Possibly after birth, the posterior attachment of the abdominal esophagus seemed to develop to the major part of the phrenoesophageal membrane with modification from the increased mass of the left fetal adrenal.
We observed that the abdominal esophagus became attached to or fused with the left adrenal at the 7th week but this connection became detached by the peritoneal recess at the 10th-18th week. This attachment seemed to be used as a course of the developing posterior gastric artery, an artery reported to be present in 55.7% of patients, as assessed by angiography [19]. Whether the fetal abdominal esophagus is attached or detached was apparently dependent on the size of the left adrenal. However, because a limited number of specimens was examined, we could not rule out the possibility that the fusion between the adrenal and abdominal esophagus at the 7th week was an individual variation. A similar situation, suggesting variations in adrenal size, was described in the development of the ligament of Treitz [6]. Therefore, the development of the posterior gastric artery was apparently dependent on the fetal anatomy of the left adrenal. After postnatal degeneration of the fetal adrenal, the abdominal esophagus might again obtain a mesentery. Finally, in contrast to adult morphology, the liver caudate lobe was located close to the abdominal esophagus in fetuses. This finding was consistent with our previous description of the fetal liver caudate lobe extending into the omental bursa [20].
Figures and Tables
Fig. 1
Sagittal sections of a 10-mm crown-rump length embryo (approximately 5 weeks). Panels (A) and (C) are the right- and left-most sides of the figure, respectively. Intervals between panels are 0.1 mm. Panel (A) displays an almost entire course of the esophagus (ES). The esophagus and aorta (AO) are embedded in posterior mesenchymal tissue continuous with the vertebral column. (B) Since the tracheal bifurcation almost corresponds to the caption bronchus (BR), a short distance between the bifurcation and stomach is evident. (C) The stomach is just below the left lung (LL) and is separated from the liver (L) and mesonephros (M) by a peritoneal recess (black stars). The space with open stars corresponds to the future superior recess of the omental bursa (OB). The major part of the OB starts to develop in the dorsal mesogastrium (B, C). AO in panel (C) indicates the left- and right-sided aortas as well as the fourth pharyngeal arch artery. DP, dorsal pancreas; DRG, dorsal root ganglia; H, heart; LCCV, left common cardinal vein; LX, larynx; PX, pharynx; SC, spinal cord; ST, stomach; TR, trachea; VC, vertebral column. All panels were prepared at the same magnification. Scale bar=1 mm.

Fig. 2
Horizontal sections of a 13-mm crown-rump leng th embr yo (approximately 5 weeks). Panels (A) and (G) are the most superior and inferior sides of the figure, respectively. Intervals between panels are 0.1 mm (A, B), 0.4 mm (B, C), 0.2 mm (C, D) and 0.1 mm (D-G). Panels (A) and (B) show levels immediately below the tracheal bifurcation: the esophagus (ES) is located on the anterior side of the aorta (AO). The asterisk in panel (A) indicates the apex of the pleural cavity. The umbilical vein (UV) runs upward along the inferior part of the pericardium (C). The diaphragm has not yet developed and the pleural and peritoneal cavities communicate via the pleuroperitoneal canal. Thus, in panel (D), the left lung (LL) faces the adrenal (AD) and liver (L) without interrupting any membranous structure. (C-E) The esophagus is connected with the aorta by a mesentery-like structure (i.e., the mesoesophagus; arrow). The pneumatoenteric recesses (PER; recesses of the pleuroperitoneal canal) are present at the inferior end of the mesoesophagus (F, G). The stomach (ST) is separated from the liver (L) by peritoneal recesses (black stars in panels D-G): and by the future omental bursa (OB, open stars). A terminal portion of the UV does not simply correspond to the inferior vena cava due to a lack of communication with the adrenal vein [5]. BR, bronchus; H, heart; M, mesonephros; PC, pericardial cavity; RL, right lung ; ST, stomach; VC, vertebral column; VN, vagus nerve. All panels were prepared at the same magnification. Scale bar=1 mm.
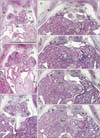
Fig. 3
Sag ittal sections of a 22-mm crown-rump leng th embr yo (approximately 7 weeks). Panels (A) and (F) are the left- and right-most sides of the figure, respectively. Intervals between panels are 0.1 mm (A, B), 0.3 mm (B, C) and 0.1 mm (C-F). Panels (A-E) were prepared at the same magnification, while panel (F) is at a higher magnification to show details of the fascia and space. Scale bar=1 mm. Panels (A) and (B) contain the right end of the left lung (LL), which is connected to the esophagus (ES) by a mesentery-like structure (open stars). In panel (C), the loose mesenchymal tissue (black stars) between the esophagus and aorta (AO) corresponds to the mesoesophagus. A recess of the peritoneal cavity (arrows) separates the stomach (ST) and abdominal esophagus from the adrenal (AD) and celiac ganglion (GL). The diaphragm (DI) has not yet extended to the space behind the AD (asterisks in panels A and B). At the superior aspect of the diaphragm, the esophagus is surrounded by a recess of the pleural cavity (arrowheads in panels C-F). The liver caudate lobe (LC) protrudes into the omental bursa (OB) in panels (D-F). DP, dorsal pancreas; G, gonad; H, heart; IVC, inferior vena cava; K, kidney; L, liver; LCCV, left common cardinal vein; M, mesonephros; PC, pericardial cavity; SC, spinal cord; SPN, splanchnic nerve; TR, trachea; UV, umbilical vein; VC, vertebral column; VN, vagus nerve.
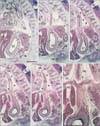
Fig. 4
Horizontal sections of a 22-mm crown-rump length embryo (approximately 7 weeks). Panels (A) and (E) are the most superior and inferior sides of the figure, respectively. The intervals between panels are 0.9 mm (A, B), 0.8 mm (B, C), 0.2 mm (C, D), and 0.1 mm (D, E). Panel (A) shows a level immediately below the tracheal bifurcation, with the esophagus (ES) located on the right side of the aorta (AO). In panels (B) and (C), the esophagus is connected with the aorta by the mesoesophagus (arrows). However, in panel (D), the mesoesophagus (arrows) is interrupted by the diaphragm. In panels (D) and (E), the adrenal (AD) is separated from the stomach (ST) or abdominal esophagus by a recess of the peritoneal cavity (arrowheads). The liver caudate lobe (LC) protrudes into the omental bursa (OB). BR, bronchus; DI, diaphragm; ES, esophagus; GB, gallbladder; H, heart; IVC, inferior vena cava; L, liver; LL, left lung; PC, pericardial cavity; PV, portal vein; RL, right lung; VN, vagus nerve. All panels were prepared at the same magnification. Scale bar=1 mm.
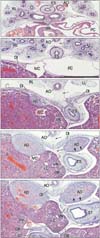
Fig. 5
Sagittal sections of a 56-mm crown-rump leng th fetus (approximately 10 weeks). Panels (A) and (E) are the right- and left-most sides of the figure, respectively. Intervals between panels are 1.1 mm (A, B), 0.3 mm (B, C), 0.9 mm (C, D), and 0.1 mm (D, E). In panels (A) and (B), the liver caudate lobe (LC) protrudes into the omental bursa (OB). In panels (B) and (C), the esophagus is attached to the abdominal aspect of the diaphragm (DI); the interface contains a fascia (black stars) that appears to extend downward between the pancreas (P) and adrenal (AD). In panels (D) and (E), a recess of the peritoneal cavity (arrowheads) is surrounded by the AD, the stomach and the dorsal mesogastrium (open stars). AD, adrenal gland (fetal adrenal cortex); AO, aorta; ES, esophagus; J, jejunum; L, liver; LL, left lung ; OB, omental bursa; P, pancreas; SP, spleen; SPA, splenic artery; ST, stomach; TC, transverse colon; VC, vertebral column; VN, vagus ner ve. All panels were prepared at the same magnification. Scale bar=1 mm.
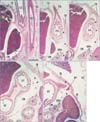
Fig. 6
Tilted horizontal sections of a 155-mm crown-rump length fetus (approximately 18 weeks). Panels (A) and (E) are the most superior and inferior sides of the figure, respectively. Intervals between panels are 16.5 mm (A, B), 2.8 mm (B, C), 2.4 mm (C, D), and 1.6 mm (D, E). Panel (A) shows a level immediately below the tracheal bifurcation, with the esophagus (ES) located on the right side of the aorta (AO). In panels (B) and (C), the mesoesophagus (arrows) connects the aorta and esophagus. Arrowheads in panel (B) indicate bilateral recesses of the pleural cavity in the pulmonary ligament. The abdominal esophagus is attached to the diaphragm (asterisk in panel D). A recess of the peritoneal cavity (open stars in panel C), present between the stomach and left adrenal, provides a sac-like enlargement (open star in panel D). Another slit-like recess is also seen (arrows in panels D and E). The liver caudate lobe (LC) protrudes into the omental bursa (OB). AD, adrenal gland (fetal adrenal cortex); BR, bronchus; DI, diaphragm; IVC, inferior vena cava; L, liver; LC, liver caudate lobe; LL, left lung; RL, right lung; ST, stomach; TH, thymus. All panels were prepared at the same magnification. Scale bar=1 mm.
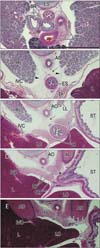
Fig. 7
A schematic representation showing recesses of the celomic, pleural and peritoneal cavities protruding into the mesoesophagus. Panel (A) (at and before 5 weeks) exhibits a primitive form in which the esophagus is embedded in a posterior mesenchymal tissue. Panel (B) (5 weeks) displays an initial mesoesophagus with the pneumatoenteric recess (a curved arrow with a): the mesentery-like shape is sculptured by the developing lungs. Panel (C) (7-18 weeks) shows a pleural recess around the lower thoracic esophagus (a curved arrow with b) as well as a peritoneal recess behind the abdominal esophagus (a curved arrow with c) between the left adrenal (AD) and stomach (ST). Asterisk indicates disappearance of the posterior mesenchymal tissue, including the mesoesophagus, due to cell death. Those recesses are similar to a Korean traditional symbol of the nature in shape, i.e., Taegeuk, as shown in the lower part of the figure.
Acknowledgements
This study was supported by a grant (0620220-1) from the National R & D Program for Cancer Control, Ministry of Health & Welfare, Republic of Korea.
References
1. Hamilton WJ, Mossman HW. Human embryology: prenatal development of form and function. 4th ed. Lodon: Williams & Wilkins;1978. p. 334–337.
2. Hayashi S, Fukuzawa Y, Rodríguez-Vázquez JF, Cho BH, Verdugo-López S, Murakami G, Nakano T. Pleuroperitoneal canal closure and the fetal adrenal gland. Anat Rec (Hoboken). 2011; 294:633–644.
3. Jin ZW, Nakamura T, Yu HC, Kimura W, Murakami G, Cho BH. Fetal anatomy of peripheral lymphatic vessels: a D2-40 immunohistochemical study using an 18-week human fetus (CRL 155 mm). J Anat. 2010; 216:671–682.
4. Kanagasuntheram R. Development of the human lesser sac. J Anat. 1957; 91:188–206.
5. Jin ZW, Cho BH, Murakami G, Fujimiya M, Kimura W, Yu HC. Fetal development of the retrohepatic inferior vena cava and accessory hepatic veins: Re-evaluation of the Alexander Barry's hypothesis. Clin Anat. 2010; 23:297–303.
6. Yang JD, Ishikawa K, Hwang HP, Yu HC, Rodríguez-Vázquez JF, Murakami G, Cho BH. Morphology of the ligament of Treitz likely depends on its fetal topographical relationship with the left adrenal gland and liver caudate lobe as well as the developing lymphatic tissues: a histological study using human fetuses. Surg Radiol Anat. 2013; 35:25–38.
7. Perlemuter L, Waligora J. Cahiers d'anatomie. Vol. 6. Thorax. 3rd ed. Paris: Masson;1976.
8. Miyake N, Takeuchi H, Cho BH, Murakami G, Fujimiya M, Kitano H. Fetal anatomy of the lower cervical and upper thoracic fasciae with special reference to the prevertebral fascial structures including the suprapleural membrane. Clin Anat. 2011; 24:607–618.
9. Boutelier P, Lefort R. Anatomic study of the abdominal mesoesophagus. Surgical deductions. J Chir (Paris). 1970; 100:371–384.
10. Cho BH, Kimura W, Song CH, Fujimiya M, Murakami G. An investigation of the embryologic development of the fascia used as the basis for pancreaticoduodenal mobilization. J Hepatobiliary Pancreat Surg. 2009; 16:824–831.
11. Hayashi S, Murakami G, Ohtsuka A, Itoh M, Nakano T, Fukuzawa Y. Connective tissue configuration in the human liver hilar region with special reference to the liver capsule and vascular sheath. J Hepatobiliary Pancreat Surg. 2008; 15:640–647.
12. Yang JD, Ishikawa K, Hwang HP, Park DE, Song JS, Fujimiya M, Murakami G, Cho BH. Retropancreatic fascia is absent along the pancreas facing the superior mesenteric artery: a histological study using elderly donated cadavers. Surg Radiol Anat. 2013; 35:403–410.
13. Watanabe Y, Lister J. Development of the human fetal phrenoesophageal membrane and its role in the anti-reflux mechanism. Surg Today. 1993; 23:722–727.
14. Kinoshita H, Umezawa T, Omine Y, Kasahara M, Rodríguez-Vázquez JF, Murakami G, Abe S. Distribution of elastic fibers in the head and neck: a histological study using late-stage human fetuses. Anat Cell Biol. 2013; 46:39–48.
15. St Peter SD, Barnhart DC, Ostlie DJ, Tsao K, Leys CM, Sharp SW, Bartle D, Morgan T, Harmon CM, Georgeson KE, Holcomb GW 3rd. Minimal vs extensive esophageal mobilization during laparoscopic fundoplication: a prospective randomized trial. J Pediatr Surg. 2011; 46:163–168.
16. Kwok H, Mariz Y, Al-Ali S, Windsor JA. Phrenoesophageal ligament re-visited. Clin Anat. 1999; 12:164–170.
17. Apaydin N, Uz A, Evirgen O, Loukas M, Tubbs RS, Elhan A. The phrenico-esophageal ligament: an anatomical study. Surg Radiol Anat. 2008; 30:29–36.
18. Nakajima F, Murakami G, Ohyama S, Horiguchi T, Sakakura Y, Yajima T, Hirata K. Potential fascial dome made by the upper leaf of the phreno-esophageal membrane. Okajimas Folia Anat Jpn. 2001; 77:201–209.
19. Okabayashi T, Kobayashi M, Sucgimoto T, Ohara S, Okamoto K, Matsuura K, Araki K. Posterior gastric artery in angiograms and its surgical importance. Hepatogastroenterology. 2005; 52:298–301.
20. Hwang SE, Cho BH, Hirai I, Kim HT, Kim JH, Fujimiya M, Murakami G, Kimura W. Topographical anatomy of Spiegel’s lobe and its adjacent organs in mid-term fetuses: its implication on the development of the lesser sac and adult morphology of the upper abdomen. Clin Anat. 2010; 23:712–719.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download