1. Brown RS Jr, Russo MW, Lai M, Shiffman ML, Richardson MC, Everhart JE, Hoofnagle JH. A survey of liver transplantation from living adult donors in the United States. N Engl J Med. 2003; 348:818–825.
2. Suehiro T, Ninomiya M, Shiotani S, Hiroshige S, Harada N, Ryosuke M, Soejima Y, Shimada M, Sugimachi K. Hepatic artery reconstruction and biliary stricture formation after living donor adult liver transplantation using the left lobe. Liver Transpl. 2002; 8:495–499.
3. Holbert BL, Baron RL, Dodd GD 3rd. Hepatic infarction caused by arterial insufficiency: spectrum and evolution of CT findings. AJR Am J Roentgenol. 1996; 166:815–820.
4. Michels NA. Newer anatomy of the liver and its variant blood supply and collateral circulation. Am J Surg. 1966; 112:337–347.
5. Couinaud C. Le foie. Etudes anatomiques et chirurgicales. Paris: Masson;1957.
6. Bismuth H. Surgical anatomy and anatomical surgery of the liver. World J Surg. 1982; 6:3–9.
7. Saxena R, Zucker SD, Crawford JM. Anatomy and physiology of the liver. In : Zakim D, Boyer TD, editors. Hepatology: A Textbook of Liver Disease. 4th ed. Philadelphia: WB Saunders;2003. p. 3–30.
8. Hjortsjo CH. The topography of the intrahepatic duct systems. Acta Anat (Basel). 1951; 11:599–615.
9. Mizumoto R, Suzuki H. Surgical anatomy of the hepatic hilum with special reference to the caudate lobe. World J Surg. 1988; 12:2–10.
10. O'Rahilly R, Muller F. Human embryology and teratology. 3rd ed. New York: Wiley-Liss;1966. p. 266.
11. Descomps P. Le tronc coeliaque. Paris: G. Steinheil;1910.
12. Piquand G. Recherches sur l'anatomie du troce coeliaque et de ses branches. Bibliogr Anat. 1910; 19:159–201.
13. Rio-Branco P. Essai sur l'anatomie et la medecine operatoire du tronc coeliaque et de ses branches, de l'artere hepatique en particulier. Paris: G. Steinheil;1912.
14. Adachi B. Das Arteriensystem der Japaner. Vol. 2. Kyoto: Maruzen;1928. p. 18–71.
15. Michels NA. Blood supply and anatomy of the upper abdominal organs with a descriptive atlas. Philadelphia: Lippincott Co.;1955.
16. Katsume Y, Kanamaru E, Sakai K. The statistical report about thirteen anomalous cases on the branches of the celiac trunk. J Kur MA. 1978; 41:266–273.
17. Ugurel MS, Battal B, Bozlar U, Nural MS, Tasar M, Ors F, Saglam M, Karademir I. Anatomical variations of hepatic arterial system, coeliac trunk and renal arteries: an analysis with multidetector CT angiography. Br J Radiol. 2010; 83:661–667.
18. Chitra R. Clinically relevant variations of the coeliac trunk. Singapore Med J. 2010; 51:216–219.
19. Prakash , Rajini T, Mokhasi V, Geethanjali BS, Sivacharan PV, Shashirekha M. Coeliac trunk and its branches: anatomical variations and clinical implications. Singapore Med J. 2012; 53:329–331.
20. Hiatt JR, Gabbay J, Busuttil RW. Surgical anatomy of the hepatic arteries in 1000 cases. Ann Surg. 1994; 220:50–52.
21. Nayak SB, Vasudeva SK. Vascular variations of liver and gallbladder: a case report. Anat Cell Biol. 2013; 46:217–219.
22. Healey JE Jr, Schroy PC, Sorensen RJ. The intrahepatic distribution of the hepatic artery in man. J Int Coll Surg. 1953; 20:133–148.
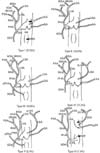
23. Onishi H, Kawarada Y, Das BC, Nakano K, Gadzijev EM, Ravnik D, Isaji S. Surgical anatomy of the medial segment (S4) of the liver with special reference to bile ducts and vessels. Hepatogastroenterology. 2000; 47:143–150.
24. Futara G, Ali A, Kinfu Y. Variations of the hepatic and cystic arteries among Ethiopians. Ethiop Med J. 2001; 39:133–142.
25. Kishi Y, Sugawara Y, Kaneko J, Akamatsu N, Imamura H, Asato H, Kokudo N, Makuuchi M. Hepatic arterial anatomy for right liver procurement from living donors. Liver Transpl. 2004; 10:129–133.
26. Wang S, He X, Li Z, Peng Z, Tam NL, Sun C, Hu A, Huang J. Characterization of the middle hepatic artery and its relevance to living donor liver transplantation. Liver Transpl. 2010; 16:736–741.
27. Yoshimura H, Uchida H, Ohishi H, Honda N, Ohue S, Kinoshita Y, Katsuragi M, Matuso N, Hosogi Y, Hatakeyama M. Evaluation of "M-point" in hepatic artery to identify left medial segment of liver. Angiographic study. Eur J Radiol. 1986; 6:195–198.
28. Kamel IR, Kruskal JB, Pomfret EA, Keogan MT, Warmbrand G, Raptopoulos V. Impact of multidetector CT on donor selection and surgical planning before living adult right lobe liver transplantation. AJR Am J Roentgenol. 2001; 176:193–200.
29. Jin GY, Yu HC, Lim HS, Moon JI, Lee JH, Chung JW, Cho BH. Anatomical variations of the origin of the segment 4 hepatic artery and their clinical implications. Liver Transpl. 2008; 14:1180–1184.
30. Daseler EH, Anson BJ. The cystic artery and constituents of the hepatic pedicle: a study of 500 specimens. Surg Gynecol Obstet. 1947; 85:47–63.
31. Koops A, Wojciechowski B, Broering DC, Adam G, Krupski-Berdien G. Anatomic variations of the hepatic arteries in 604 selective celiac and superior mesenteric angiographies. Surg Radiol Anat. 2004; 26:239–244.
32. Couinaud C. Le foie. Etudes anatomiques et chirurgicales. Paris: Masson & Cie;1954. p. 146–186.
33. Couinaud C. Surgical anatomy of the liver revisited: embryology. Paris: Couinaud;1989. p. 11–24.
34. Fan ST, Lo CM, Liu CL, Wang WX, Wong J. Safety and necessity of including the middle hepatic vein in the right lobe graft in adult-to-adult live donor liver transplantation. Ann Surg. 2003; 238:137–148.
35. Sakamoto Y, Takayama T, Nakatsuka T, Asato H, Sugawara Y, Sano K, Imamura H, Kawarasaki H, Makuuchi M. Advantage in using living donors with aberrant hepatic artery for partial liver graft arterialization. Transplantation. 2002; 74:518–521.
36. Aramaki O, Sugawara Y, Kokudo N, Takayama T, Makuuchi M. Branch patch reconstruction in living donor liver transplantation: arterialization of grafts with replaced type arteries. Transplantation. 2006; 82:1541–1543.
37. Sakai H, Okuda K, Yasunaga M, Kinoshita H, Aoyagi S. Reliability of hepatic artery configuration in 3D CT angiography compared with conventional angiography: special reference to living-related liver transplant donors. Transpl Int. 2005; 18:499–505.







 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download