Abstract
The present study investigated the cytogenetic and testicular damage induced by the antiepileptic drug, sodium valporate (SVP) in albino rats and the effect of saffron aqueous extracts. Treating rats with SVP caused a significant increase in the chromosomal aberrations either structural or numerical and decreased the mitotic index. Besides, animals administered SVP showed DNA damage appeared in the single strand breaks (comet assay). Testis of SVP-treated rats showed many histopathological changes. A significant decrease in seminiferous tubules and their epithelial heights diameters and inhibition of spermatogenesis was recorded. In addition, the number of sperm head abnormalities was increased. Biochemical results revealed an increase in malondialdhyde (MDA) which is lipid peroxidation marker and a significant decrease in the level of serum antioxidant enzyme, catalase (CAT) and reducing antioxidant power (RAP). Animals given SVP and saffron showed an improvement in chromosomal aberrations, mitotic index, DNA damage and testicular alterations caused by SVP. Moreover, MDA decreased and CAT and RAP increased. It is concluded from the present results that the ameliorative effects of saffron extract against SVP-induced cytogenetic and testicular damage in albino rats may be due to the presence of one or more antioxidant components of saffron.
The antiepileptic drugs are a diverse group of pharmaceuticals used in the treatment of epileptic seizures. They can be grouped according to their main mechanisms of action into sodium channel blockers, calcium current inhibitors, gamma-aminobutyric acid enhancers, glutamate blockers, carbonic anhydrase inhibitors and hormones [1]. Sodium valproate is the sodium salt of valproic acid (VPA) and is an anticonvulsant used in the treatment of epilepsy, anorexia nervosa, panic attack, anxiety disorder, posttraumatic stress disorder, migraine, and bipolar disorder, as well as other psychiatric conditions [2]. Use of valproate was found to be associated with many side effects. Kingsley et al. [3] demonstrated that sodium valproate is hepatotoxic in both humans and rat hepatocytes. Witczak et al. [4] reported that newborns of mothers exposed to valporate are at increased risk for major congenital malformations, cognitive impairment and fetal death. Jentink et al. [5] found that there is significant association between exposure of the unborn child to VPA monotherapy in the first trimester and Spina bifida, arterial septa defects, cleft palate, hypospadias, polydactyl and craniosynostosis. Isojarvi et al. [6] reported that valproate was associated with sperm abnormalities in men with epilepsy. Bairy et al. [7] concluded that sodium valproate causes reversible change in sperm motility, sperm count, morphology and cytoarchitecture of tests. Watkins et al. [8] found that subcutaneous fibrosarcomas were significantly increased in VPA treated Wistar rat males. Also they found that adenocarcinomas of the uterus and cervix were increased in calcium valproate treated females compared to controls.
Crocus sativus L., commonly known as saffron, is used in folk medicine as an anti-spasmodic, eupeptic, gingival, sedative, anti-catarrhal, carminative, diaphoretic, expectorant, stimulant, stomachic, aphrodisiac, and emmenagogue. Recent studies have demonstrated that saffron extract has anti-microbial [9], anti-convulsant [10], anti-depressant [11], and anti-inflammatory [12]. Nair et al. [13] reported the anticancer activity of saffron extract (dimethyl crocetin) against a wide spectrum of murine tumors and human leukemia cell lines. Premkumar et al. [14] studied the chemoprotective potential of saffron against the genotoxicity of three well-known anti-tumor drugs cisplatin, cyclophosphamide, and mitomycin using comet assay. Iranshahi et al. [15] reported that aqueous and ethanolic extracts of saffron exhibit hepatoprotective effects against liver damages induced by CCl4 in mice. Naghizadeh et al. [16] reported that crocin, the extract of saffron has protective effect on cisplatin-induced nephrotoxicity in rat. The present work aims to study the effect of saffron aqueous extract on sodium valporate (SVP)-induced cytogenetic and testicular alterations in albino rats.
Saffron, the dried stigmas of Crocus sativus flower were obtained from Al-alawy market, Jeddah, Saudi Arabia. One gram of saffron was soaked in 100 ml distilled water. After 2 hours it was homogenized in the same distilled water, stirred for 1 hour and filtered. The residue was re-extracted with fresh distilled water. This aqueous extract was lyophilized and stored at 4℃ until further use [14].
Depakine, sodium valporate, is available for oral administration as syrup supplied in bottles of 125 ml produced by Global Napi Pharmaceuticals, Cairo, Egypt. It is used at a dose level of 80 mg/kg body weight of rat modified according to therapeutic dose of human [17] and each animal were orally given 1 ml containing the desired dose.
Adult male albino rats (Rattus norvegicus) (150±5 g) were used in the present work. The animals were kept in special plastic cages in the animal house under constant temperature 25±1℃ with a reverse natural dark- light cycle 12/12 hours, for at least one week before the experimental work. The animals were maintained on a standard rodent diet and water was available ad libitum. Animals, experiments and housing procedures were performed in accordance to the animal care rules approved by the authorities of Menoufia University, Egypt. They were divided into 4 groups.
Group I: Animals of this group (10 rats) had been kept as controls and were given basal diet.
Group II: Animals of this group (10 rats) were administered by gavage saffron extract at a dose level of 20 mg/kg body weight, daily for 6 weeks.
Group III: Animals of this group (15 rats) were orally given SVP at a dose level of 80 mg/kg body weight daily dissolved in water for 6 weeks.
Group IV: Animals of this group (25 rats) were orally given the same dose of SVP and saffron extract for 6 weeks.
Animals were sacrificed after 6 weeks of treatment and bone marrow cells were collected for analysis of chromosomal aberrations and mitotic indices by colchicines-hypotonic technique. After completion of the treatment period, five animals from each group were i.p. injected, 2 hours before sacrifice, with 0.5 ml colchicine (3 mg/kg body weight), to increase the number of metaphase spreads. Bone marrow cells were collected from the femurs in isotonic NaCl solution and bone marrow smears were prepared. For each group, slides were stained with Giemsa and mounted in DPX. For each animal, 50 metaphase spreads were scored for chromosomal aberrations. The mitotic index was obtained by counting the number of mitotic cells in 1,000 cells/animal [18].
This technique permits the detection and an evaluation of single-stranded DNA breaks. Eukaryotic cells are embedded in agarose gel on a microscopic slide, lysed by detergents and high salt at pH 10, and then electrophoresed for damage display which shows increased migration of the DNA from the nucleus towards the anode. Low-melting temperature agarose and ultra pure a garose, Triton X-100, sodium sarcosinate, ethylenediamine-tetra acetic acid disodium salt (Na-EDTA), Trizma base and ethidium bromide were obtained from Sigma Chemical Co. (St. Louis, MO, USA). Phosphate buffered saline without calcium and magnesium, RBMI 1640 medium (Gibco-BRL, Gaithersburg, MD, USA), Ficoll separating solution and trypan-blue were used in comet assay. Examination was done with a fluorescent microscope (BX 41, Olympus, Tokyo, Japan) equipped with an excitation filter of 510 nm and barrier filter of 590 nm. The migration was evaluated by observing and measuring the nuclear DNA, and 500 spots of DNA were examined and classified into three types: 1) normal spots with round shape; 2) damaged spots in which the length of the migrated fragments was less than or equal to the diameter of the basal nuclear DNA; and 3) strongly damaged spots where the length of the comet was greater than the diameter of the basal nuclear DNA [19].
For histological studies, animals were dissected and their testes were removed and fixed in 10% neutral formalin for 24 hours, washed in running tap water for 24 hours and transferred to 70% ethyl alcohol. Tissues were dehydrated in ascending series of ethyl alcohol, cleared in xylene and embedded in wax. Sections of 5 µm thickness were cut using rotary microtome and mounted on clean slides without using any adhesive medium. Sections were stained with Ehrlich's haematoxylin, counterstained with eosin and photographed.
The sperm suspension was obtained from animals by cutting the caudal epididymis of a testis in few drops of mammalian saline. The sperm suspension was spread on clean glass slides. Sperm smears were dried in air and incubated at oven at 50℃ overnight. The sperms were fixed in methyl alcohol and stained with haematoxylin and eosin. A total of 1,000 sperms were examined for each animal directly under microscope to detect the morphological abnormalities in head region [20].
For biochemical determination, blood samples were collected from animals after 6 weeks of treatment. Sera were obtained by centrifugation of the blood sample and stored at -20℃ until assayed for the biochemical parameters. The extent of lipid peroxidation was estimated as the concentration of thiobarbituric acid reactive product (malondialdhyde) according to Ohkawa et al. [21]. Total antioxidant capacity was determined by ferric reducing antioxidant power [22]. Catalase activity was determined from the rate of decomposition of H2O2 [23].
Data were expressed as mean values±SD and statistical analysis was performed using one way ANOVA to assess significant differences among treatment groups. The criterion for statistical significance was set at P<0.05. All statistical analyses were performed using SPSS ver. 16 (SPSS Inc., Chicago, IL, USA).
Treating animals of SVP induced structural aberrations such as chromatid deletion, chromatid fragment, chromosomal ring, centromeric attenuation of chromosomes, centric fusion of chromosomes, end to end association, chromatid gaps and chromatid breaks were recorded (Table 1). Numerical aberrations such as monosomy, trisomy, tetrasomy and polyploidy were also detected (Table 2). A significant increase in these aberrations was observed after the treatment with SVP when compared with the control animals. Animals treated with SVP and saffron showed a decrease in these aberrations when compared with SVP-treated animals. Also, animals treated with SVP showed a decrease in the mitotic index when compared with the control groups and this is increased by saffron administration (Table 3).
A significant increase in DNA fragmentation appeared as damaged and strongly damaged spots appeared in lymphocytes of rats treated with SVP as detected by comet assay. Rats treated with SVP and saffron showed improvement in DNA by decreasing the number of damaged and strongly damaged spots (Table 4, Fig. 1).
Normal sperm shape composed of hook, head and tail (Fig. 2A). Sperm head abnormalities (without hook, banana type, amorphous, hummer shape) (Fig. 2B-E) were significantly increased in animals given SVP. Treating animals with SVP and saffron caused a significant decrease in sperm head abnormalities (Table 5).
Sections of control rats showed normal testicular architecture with an orderly arrangement of germinal cells (spermatogonia, spermatocytes, spermatids and spermatozoa) and Sertoli cells (Fig. 3A). Animals treated with saffron showed nearly normal histological structures as shown in the controls. Treating animals with SVP for six weeks showed a distinct histological difference when compared with the control ones. A decrease in the diameter of the tubules and enlargement of their lumens was observed. The germ cells were detached from the basal lamina and the intertubular spaces showed blood hemorrhage (Fig. 3B). The blood vessels appeared with severe congestion (Fig. 3C). The number of all types of spermatogenic cells was significantly reduced. The seminiferous tubules were found containing few spermatogonia and Sertoli cells and large number of them was degenerated and exfoliated in the lumen of the tubules (Fig. 3D). Animals given SVP with saffron showed an improvement in the histopathological alterations appeared in the treatment with SVP. The germ layers increased with appearance of somewhat normal cells as well as increase in number of sperm bundles (Fig. 3E).
A significant decrease in the diameter and germ cell height of seminiferous tubules was recorded in testes of animals treated with SVP in compared with the normal ones (Table 6). Animals treated with SVP and saffron showed marked improvement in the mean tubular diameter and in germ cell height in comparison with the SVP-treated animals.
Data in Fig. 4A-C revealed that there is no remarkable change concerning malondialdehyde, catalase (CAT), and reducing antioxidant power in sera of control rats and those treated with saffron. In contrast, elevated malondialdehyde, decrease of CAT and reducing antioxidant power was recorded in animals treated with SVP for 6 weeks. Administration of saffron resulted in a significant decrease in malondialdehyde and increase in CAT and reducing antioxidant power compared to SVP group.
The present study investigated the cytogenetic and histopathological effects of antiepileptic drug, depakine on male rats and the ameliorative role of saffron extract. Animals treated with SVP showed an increase in the mean number of structural chromosomal aberrations including chromatid deletion, chromosomal ring, centromeric attenuation, centric fusion, chromatid fragmentation, chromatid gaps , chromatid breaks and end to end association. Numerical chromosomal aberrations including monosomy, trisomy, tetrasomy and polyploidy also increased. There was a marked decrease in the mitotic index and increase in DNA damage. These results are in agreement with previous studies which reported the cytogenetic effects of SVP.
Hu et al. [24] reported that sister-chromatid exchange and chromosome aberrations were higher in peripheral lymphocytes of epileptic children treated in monotherapy with VPA for 6??2 months. Karapidaki et al. [25] found a statistically significant increase in sister chromatid exchanges frequency and a significant decrease in the proliferation rate index in lymphocytes of peripheral blood cultures from 3 healthy donors given VPA and ziprasidone. Witczak et al. [4] showed that the anti-epileptic drugs, VPA and carbamazepine analogues, given to epileptic women in mono- and poly-therapy during pregnancy evoked potentially clastogenic and genotoxic effects in cord-blood lymphocytes. Denli et al. [26] recorded a significant increase in cell damage in comet assay of peripheral lymphocytes of the epileptic patients undergoing long-term valporic acid monotherapy Felisbino et al. [27] reported that exposing HeLa cells to valporic acid caused DNA fragmentation and decrease of mitotic index. Li et al. [28] observed that both VPA and Aurora kinase inhibitor VE465, when used alone, induced apoptosis in the 2008/C13 cell line.
Results obtained in this study showed a significant decrease in seminiferous tubules and their epithelial heights diameters and inhibition of spermatogenesis were recorded in animals treated with SVP. Moreover, decrease in sperm number and increase in sperm head abnormalities was observed. Similar results were obtained in mice [29], rats [7], and men [6, 30] treated with VPA. Cansu et al. [31] studied the effects of valproate and oxcarbazepine on testicular development in rats. The results showed that testis and relative testis weights were significantly lowerd in the VPA group compared to the control group. Spermatogonia, pachytene spermatocyte and round spermatocyte numbers were decreased in all stages in both the VPA and oxcarbazepine groups compared to the control group.
Treating rats with SVP induced a significant increase in, malondialdhyde which is lipid peroxidation marker and a significant decrease in the level of serum antioxidant enzyme, CAT, and reducing antioxidant power. Similarly, Vidya and Subramanian [32] reported that SVP-treatment was found to increase levels of malondialdhyde and hydroperoxides and decreased levels of enzymatic (superoxide dismutase [SOD], CAT, and glutathione peroxidase [GPx]) and non-enzymatic (glutathione [GSH]) antioxidants in rats. The activities of SOD, GPx, and GSH reductase were insignificantly lower, whereas the malondialdehyde concentration was insignificantly higher in the erythrocytes of children with epilepsy treated with SVP [33]. Zhang et al. [34] found that the levels of malondialdehyde in neutrophils of SVP-treated patients were higher while the activities of SOD and CAT were significantly lower than the control and untreated groups. Thus, the cytogenetic and testicular damage induced in rats in the present work may be attributed to the oxidative stress generated by SVP.
The current results showed that saffron extract improved the cytogenetic alterations induced by VPA. Hosseinzadeh and Sadeghnia [35] examined the effect of aqueous extract of Crocus sativus stigmas and crocin (trans-crocin 4) on methyl methanesulfonate (MMS)-induced DNA damage in multiple mice organs using the comet assay. A significant increase in the % tail DNA was seen in nuclei of different organs of MMS-treated mice. Pretreatment with saffron extract only reduced DNA damage in liver, lung, kidney, and spleen. Premkumar et al. [14] reported that pre-treatment with saffron significantly inhibited anti-tumor drugs (cisplatin, cyclophosphamide, and mitomycin C) induced cellular DNA damage as revealed by decreased comet tail length, tail moment and percent DNA in the tail.
The present results revealed that saffron extract ameliorates the testicular damage, sperm count and abnormalities induced by SVP in albino rats. Similarly, Asadi et al. [36] reported that saffron extract improved semen parameters (sperm concentration, motility and viability in cauda of epididymis) in rats exposed to cadmium. Heidary et al. [37] found that prescribing edible saffron is effective on increasing the average number and motility of sperms in nonsmoker infertile men with oligospermia. Modaresi et al. [38] reported that in mice, saffron consumption with 100 mg/kg dosage during 20 days resulted in increased serum levels of follicle stimulating hormone, luteinizing hormone, and testosterone.
Results obtained in the present work showed that saffron extract caused a significant decrease in malondialdhyde and a significant increase in the level of serum antioxidant enzyme, CAT, and reducing antioxidant power as a result of SVP administration. The antioxidant effects of saffron and its extracts were reported under the effect of different toxins. Goli et al. [39] concluded that saffron petal as the main by-product of saffron production possessed considerable phenolic compounds which showed high antioxidant power. Hosseinzadeh et al. [40] reported that safranal and crocin, extracts of saffron, have antioxidant effects. Treating mice exposed to ALCL3 by saffron improved the disrupted liver biochemical markers and alleviated the increase of lipid peroxidation [41]. Vakili et al. [42] indicated that crocin has protective effects against ischemic reperfusion injury and cerebral edema in a rat model of stroke and significantly reduced malondialdhyde content and increased activity of SOD and GPx in the ischemic cortex. Mohajeri and Doustar [43] found that ethanolic extract of saffron had the ability to reduce lipid peroxidation, and improved the antioxidant enzyme activities, SOD, CAT, and GSH-related enzymes in liver of rats treated with cisplatin. Giaccio [44] reported that crocetin, protects against AFB1-induced hepatotoxicity and oxidation damage in rats with increase of glutathion-S-transferase. Treating mice with saffron was found to improve learning and memory, accompanied by reduced lipid peroxidation products, higher total brain antioxidant activity and reduced caspase-3 activity [45].
The chemical composition of stigmas of Crocus sativus L. has been investigated in several studies during the past two decades. Reportedly, stigma of Crocus sativus flower contains three main metabolites; crocins, picrocrocins and safranal in addition to anthocyanins, flavonoids, vitamins (riboflavin and thiamine), amino acids, proteins, starch, mineral matter, gums, and other chemical compounds [46]. It was reported that crocin showed a high radical scavenging activity (50% and 65% for 500 and 1,000 ppm solution in methanol, respectively), followed by safranal (34% for 500 ppm solution) [47]. It is concluded from the present results that the ameliorative effects of saffron extract against SVP-induced cytogenetic and testicular damage in albino rats may be due to the presence of one or more antioxidant components of saffron.
Figures and Tables
Fig. 1
Single strand breaks (comet assay) of DNA of rat lymphocytes. (A) Normal DNA spots (no migration). (B) Damaged DNA spots (migration towards the anode). (C) Strong damaged DNA spots (more migration towards the anode).

Fig. 2
(A) Normal head of a sperm with hook (arrow). (B) Without hook. (C) Banana type. (D) Amorphous. (E) Hummer-shape (A-E, ×1,000).
Fig. 3
(A) Section in testis of a control rat. (B) Testis of sodium valporate (SVP)-treated rat showing interstitial hemorrhage (H) and detachment os spermatogenic cells (arrows). (C) Congested blood vessel (arrow). (D) Exfoliation of germ cells in the lumen of the tubule (arrow) and reduction of spermatogenic cells. (E) Testis of a rat treated with SVP+ saffron showing an improvement in histological appearance and increase of germ cells (A-E, H&E, ×400). IT, interstitial tissue; S, sperms; SC, spermatocytes; Sg, spermatogonia; Sp, sperm bundles.
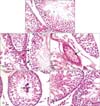
Fig. 4
(A) Effect of different treatments on malondialdhyde (MDA). (B) Effect of different treatments on catalase (CAT). (C) Effect of different treatments on reducing antioxidant power (RAP). a)Significant at P<0.05 compared with control group. b)Significant at P<0.05 compared with sodium valporate (SVP) group.

Table 1
Average of structural chromosomal abnormalities observed in bone marrow cells of rats treated with SVP and/or saffron

Table 2
Average of numerical chromosomal abnormalities observed in bone marrow cells of rats treated with SVP and/or saffron

Table 3
The mean value of mitotic index in bone marrow cells of rats treated with SVP and/or saffron

Table 4
Mean value of DNA damage detected by comet assay in rat lymphocytes treated with SVP and/or saffron

References
1. Gelder M, Mayou R, Geddes J. Psychiatry. 3rd ed. Oxford: Oxford University Press;2006.
2. Löscher W. Basic pharmacology of valproate: a review after 35 years of clinical use for the treatment of epilepsy. CNS Drugs. 2002; 16:669–694.
3. Kingsley E, Gray P, Tolman KG, Tweedale R. The toxicity of metabolites of sodium valproate in cultured hepatocytes. J Clin Pharmacol. 1983; 23:178–185.
4. Witczak M, Kociszewska I, Wilczyński J, Lopaczyńska D, Ferenc T. Evaluation of chromosome aberrations, sister chromatid exchange and micronuclei in cultured cord-blood lymphocytes of newborns of women treated for epilepsy during pregnancy. Mutat Res. 2010; 701:111–117.
5. Jentink J, Loane MA, Dolk H, Barisic I, Garne E, Morris JK, de Jong-van den Berg LT. EUROCAT Antiepileptic Study Working Group. Valproic acid monotherapy in pregnancy and major congenital malformations. N Engl J Med. 2010; 362:2185–2193.
6. Isojarvi JI, Lofgren E, Juntunen KS, Pakarinen AJ, Päivänsalo M, Rautakorpi I, Tuomivaara L. Effect of epilepsy and antiepileptic drugs on male reproductive health. Neurology. 2004; 62:247–253.
7. Bairy L, Paul V, Rao Y. Reproductive toxicity of sodium valproate in male rats. Indian J Pharmacol. 2010; 42:90–94.
8. Watkins JR, Gough AW, McGuire EJ, Goldenthal E, de la Iglesia FA. Calcium valproate-induced uterine adenocarcinomas in Wistar rats. Toxicology. 1992; 71:35–47.
9. Vahidi H, Kamalinejad M, Sedaghati N. Antimicrobial properties of Crocus sativus L. Iran J Pharm Res. 2002; 1:33–35.
10. Hosseinzadeh H, Khosravan V. Anticonvulsant effects of aqueous and ethanolic extracts of Crocus sativus L. stigmas in mice. Arch Iran Med. 2002; 5:44–47.
11. Hosseinzadeh H, Karimi G, Niapoor M. Antidepressant effects of Crocus sativus L. stigma extracts and their constituents, crocin and safranal, in mice. Acta Hortic. 2004; 650:435–445.
12. Hosseinzadeh H, Younesi HM. Antinociceptive and anti-inflammatory effects of Crocus sativus L. stigma and petal extracts in mice. BMC Pharmacol. 2002; 2:7.
13. Nair SC, Pannikar B, Panikkar KR. Antitumour activity of saffron (Crocus sativus). Cancer Lett. 1991; 57:109–114.
14. Premkumar K, Abraham SK, Santhiya ST, Ramesh A. Protective effects of saffron (Crocus sativus Linn.) on genotoxins-induced oxidative stress in Swiss albino mice. Phytother Res. 2003; 17:614–617.
15. Iranshahi M, Khoshangosht M, Mohammadkhani Z, Karimi G. Protective effects of aqueous and ethanolic extracts of saffron stigma and petal on liver toxicity induced by carbon tetrachloride in mice. Pharmacologyonline. 2011; 1:203–212.
16. Naghizadeh B, Boroushaki MT, Vahdati Mashhadian N, Mansouri MT. Protective effects of crocin against cisplatin-induced acute renal failure and oxidative stress in rats. Iran Biomed J. 2008; 12:93–100.
17. Paget GE, Barnes JM. Toxicity tests. In : Laurence DR, Bacharach AL, editors. Evaluation of Drug Activities Pharmacometries. London and New York: Academic Press;1964. p. 135.
18. Kashiwada E, Kuroda K, Endo G. Aneuploidy induced by dimethylarsinic acid in mouse bone marrow cells. Mutat Res. 1998; 413:33–38.
19. Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988; 175:184–191.
20. Wyrobek AJ, Bruce WR. Chemical induction of sperm abnormalities in mice. Proc Natl Acad Sci U S A. 1975; 72:4425–4429.
21. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979; 95:351–358.
22. Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of "antioxidant power": the FRAP assay. Anal Biochem. 1996; 239:70–76.
23. Aebi H, Wyss SR, Scherz B, Skvaril F. Heterogeneity of erythrocyte catalase II. Isolation and characterization of normal and variant erythrocyte catalase and their subunits. Eur J Biochem. 1974; 48:137–145.
24. Hu LJ, Lu XF, Lu BQ, Huang YQ. The effect of valproic acid on SCE and chromosome aberrations in epileptic children. Mutat Res. 1990; 243:63–66.
25. Karapidaki I, Ekonomopoulou MT, Akritopoulou K, Anestakis D, Iakovidou-Kritsi Z. Cytogenetic effects of valproic acid and ziprasidone in human lymphocyte cultures. Neuropsychobiology. 2011; 64:219–223.
26. Denli M, Aydin HI, Döndaröz R, Özişik T, Erdem E, Baltaci V. Genotoxicity evaluation in female patients on valproic acid monotherapy using alkaline single cell gel electrophoresis (comet assay). East J Med. 2000; 5:61–65.
27. Felisbino MB, Tamashiro WM, Mello ML. Chromatin remodeling, cell proliferation and cell death in valproic acid-treated HeLa cells. PLoS One. 2011; 6:e29144.
28. Li Y, Liu T, Ivan C, Huang J, Shen DY, Kavanagh JJ, Bast RC Jr, Fu S, Hu W, Sood AK. Enhanced cytotoxic effects of combined valproic acid and the aurora kinase inhibitor VE465 on gynecologic cancer cells. Front Oncol. 2013; 3:58.
29. Khan S, Ahmad T, Parekh CV, Trivedi PP, Kushwaha S, Jena G. Investigation on sodium valproate induced germ cell damage, oxidative stress and genotoxicity in male Swiss mice. Reprod Toxicol. 2011; 32:385–394.
30. Roste LS, Taubøll E, Haugen TB, Bjornenak T, Saetre ER, Gjerstad L. Alterations in semen parameters in men with epilepsy treated with valproate or carbamazepine monotherapy. Eur J Neurol. 2003; 10:501–506.
31. Cansu A, Ekinci O, Serdaroglu A, Gürgen SG, Ekinci O, Erdogan D, Coskun ZK, Tunc L. Effects of chronic treatment with valproate and oxcarbazepine on testicular development in rats. Seizure. 2011; 20:203–207.
32. Vidya M, Subramanian S. Effects of micro-ketoglutarate on antioxidants and lipid peroxidation products in rats treated with sodium valproate. J Appl Biomed. 2006; 4:141–146.
33. Sobaniec W, Solowiej E, Kulak W, Bockowski L, Smigielska-Kuzia J, Artemowicz B. Evaluation of the influence of antiepileptic therapy on antioxidant enzyme activity and lipid peroxidation in erythrocytes of children with epilepsy. J Child Neurol. 2006; 21:558–562.
34. Zhang YJ, Zhang M, Wang XC, Yu YH, Jin PJ, Wang Y. Effects of sodium valproate on neutrophils\' oxidative metabolism and oxidant status in children with idiopathic epilepsy. Zhonghua Er Ke Za Zhi. 2011; 49:776–781.
35. Hosseinzadeh H, Sadeghnia HR. Effect of safranal, a constituent of Crocus sativus (saffron), on methyl methanesulfonate (MMS)-induced DNA damage in mouse organs: an alkaline single-cell gel electrophoresis (comet) assay. DNA Cell Biol. 2007; 26:841–846.
36. Asadi MH, Zafari F, Sarveazad A, Abbasi M, Safa M, Koruji M, Yari A, Alizadeh Miran R. Saffron improves epididymal sperm parameters in rats exposed to cadmium. Nephrourol Mon. 2014; 6:e12125.
37. Heidary M, Vahhabi S, Reza Nejadi J, Delfan B, Birjandi M, Kaviani H, Givrad S. Effect of saffron on semen parameters of infertile men. Urol J. 2008; 5:255–259.
38. Modaresi M, Mesripour M, Asadi Margh, Hamedanian MK. Effect of Saffron (Crocus sativus) extract on level of FSH, LH and testosterone in mice. J Zanjan Univ Med Sci Health Serv. 2008; 16:11–17.
39. Goli SA, Mokhtari F, Rahimmalek M. Phenolic compounds and antioxidant activity from Saffron (Crocus sativus L.) Petal. J Agric Sci. 2012; 4:175–180.
40. Hosseinzadeh H, Shamsaie F, Mehri S. Antioxidant activity of aqueous and ethanolic extracts of Crocus sativus L. stigma and its bioactive constituent, crocin and safranal. Pharmacogn Mag. 2009; 5:419–424.
41. Shati AA, Alamri SA. Role of saffron (Crocus sativus L.) and honey syrup on aluminum-induced hepatotoxicity. Saudi Med J. 2010; 31:1106–1113.
42. Vakili A, Einali MR, Bandegi AR. Protective effect of crocin against cerebral ischemia in a dose-dependent manner in a rat model of ischemic stroke. J Stroke Cerebrovasc Dis. 2014; 23:106–113.
43. Mohajeri D, Doustar Y. Protective effect of ethanolic extract of Crocus sativus L. (saffron) stigma against cisplatin induced hepatotoxicity in rats. Med Sci J Islamic Azad Univ. 2012; 21:251–261.
44. Giaccio M. Crocetin from saffron: an active component of an ancient spice. Crit Rev Food Sci Nutr. 2004; 44:155–172.
45. Papandreou MA, Tsachaki M, Efthimiopoulos S, Cordopatis P, Lamari FN, Margarity M. Memory enhancing effects of saffron in aged mice are correlated with antioxidant protection. Behav Brain Res. 2011; 219:197–204.
46. Rios JL, Recio MC, Giner RM, Máñez S. An update review of saffron and its active constituents. Phytother Res. 1996; 10:189–193.
47. Assimopoulou AN, Sinakos Z, Papageorgiou VP. Radical scavenging activity of Crocus sativus L. extract and its bioactive constituents. Phytother Res. 2005; 19:997–1000.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download