Introduction
Cigarette smoking is a major environmental health problem that is implicated in the development of many human diseases [1]. Nicotine is the primary pharmacological component of tobacco that acts on the brain and is believed to be the addictive tobacco agent [2]. In addition, therapeutic use of nicotine was suggested due to its anti-inflammatory effects [3] and antidepressant effects [4] in addition to it's neuroprotective effect in Parkinson's disease [5].
Smoking was reported to worsen the auditory thresholds at high frequencies and to increase the incidence of tinnitus, which is a frequent symptom in cochlear dysfunction [6]. Smoking has been positively associated with hearing loss in adults [7]. Sensory hearing loss occurs when there is damage to the cochlea, the end organ of hearing that lies deep within the temporal bone [8]. Inner hair cells (IHC) and outer hair cells (OHCs) are the sensory cells of hearing and they are very sensitive to harmful influences and toxic drugs [9]. The sensory hair cells are organized along with several types of supporting cells in an ordered pattern forming the organ of Corti with their apical surfaces joined together to form the reticular lamina [10]. The effect of nicotine on sensory hearing loss is unclear.
Therefore, the aim of this work, was to investigate the structural changes of the choclea that would be induced under the influence of nicotine administration in two different doses for the same duration on the structure of the cochlea of adult male guinea pigs. Using light microscopic, transmission electron microscopic (TEM) and scanning electron microscopic (SEM) techniques, the structure of the cochlea was evaluated after one month of nicotine exposure.
Materials and Methods
Materials
The drug
Nicotine hydrogen tartrate salt [1-methyl-2-(3-pyridyl) pyrrolidine-bitartrate salt] was purchased from Sigma-Aldrich (St. Louis, MO, USA). The drug was dissolved in physiological saline (0.9% sodium chloride) and injected subcutaneously.
The animals
The experimental animal selected was pigmented guinea pigs since they are easy to handle and the cochlea is easy to dissect. In addition, subcutaneous injection of nicotine is easily made in the nape of the neck [11]. A total number of 15 adult male pigmented guinea pigs that were 4 months age, 450-600 g body weight at the start of the experiment, were used in this study. The animals were housed in stainless steel cages under standard conditions (light, temperature and free access to food and water). Animal care and use were in accordance with procedures outlined in the National Institutes of Health Guidelines. The experiment was approved by the institutional ethics committee of Assiut University. The animals were divided into two groups:
Group I (control): This group included 5 animals that were injected subcutaneously with saline daily for one month.
Group II (nicotine treated): This group included 10 guinea pigs and was further subdivided into two equal subgroups, 5 animals each:
- Subgroup IIA: injected subcutaneously with nicotine dissolved in normal saline at a dose of 3 mg/kg body weight daily for one month
- Subgroup IIB: injected subcutaneously with nicotine dissolved in normal saline at a dose of 6 mg/kg body weight daily for one month
The nicotine doses (3 mg/kg and 6 mg/kg) were chosen to approximate the plasma levels reported in moderate and heavy smokers, respectively [12].
Methods
At the end of the experiment, the animals were anaesthetized with ether; their hearts were exposed and perfused through the left ventricle with glutaraldehyde cacodylate fixative. Each temporal bone with its tympanic cavity was removed by exposing the tympanic bulla and then opened for cochlear exposure. The labyrinths were promptly removed. The two cochleae of each animal were gently perfused with glutaraldehyde cacodylate fixative through an opening made on the cochlear apex. Then, the cochleae were put in the same fixative for one day.
The basal half of the left cochleae from all animals in group I and group IIA were used for transmission electron microscopy. Cochleae were decalcified in 4% EDTA in 0.1 M cacodylate buffer pH 7.3 for 48 hours. Then, the cochleae were rinsed in 0.1 M sodium cacodylate buffer several times before processing for TEM. Semithin sections (0.5-1 µm) were stained with toluidine blue (TB) and examined with light microscope [13]. Ultrathin sections (500-800A°) were cut, contrasted with uranyl acetate and lead citrate [14] and examined with the TEM JEOL (JEM-100 CXII, JEOL, Tokyo, Japan) and photographed at 80 KV in Assiut University-Electron Microscope Unit.
The right cochleae from animals of all groups were submitted to microdissection under a dissecting microscope in order to remove the bony wall of the cochleae and expose the membranous cochleae, whereas the tectorial membranes undergo retraction during processing. A graded series of alcohols was used for dehydration and liquid carbon dioxide was used to dry the specimens. Dried specimens were mounted on aluminum stubs and sputter coated with gold [15]. A SEM JEOL (J.S.M-5400 LV, Japanese Electron Optic Laboratory, Tokyo, Japan) was used to view the specimens. Photographs of the basal turn of the cochlea of each animal were taken at 15 KV.
Results
Light microscopy
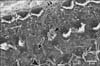
In TB-stained semithin sections of control animals, the cell bodies of supporting cells rest on the basilar membrane which underlied the entire organ of Corti. The tectorial membrane appeared as a long spiral "blanket" which covered the organ of Corti. The long inner and outer pillars extended from the basilar membrane to the apical surface of the sensory epithelium (reticular lamina) enclosing the tunnel of Corti in between. Hensen's cells were located further laterally in the organ of Corti (Fig. 1). Semithin TB-stained sections of the organ of Corti of group IIA revealed that the IHCs and OHCs had densely stained nuclei and vacuolated cytoplasm. The Hensen's cells were disorganized and extended towards the pillar cell region (Fig. 2).
Transmission electron microscopy
In ultrathin sections of control animals, the OHCs characteristically revealed electron dense cuticular plate and graded height of straight stereocilia at their apical ends and subsurface cisternae located under their lateral plasma membrane. The cell bodies of Deiters' cells, which had rounded heterochromatic nuclei and prominent bundles of microtubules running in their cytoplasm, enclosed the basal ends of OHCs and sent phalangeal processes (Figs. 3, 4). These processes expanded terminally at the reticular lamina where it formed junctional complex with the OHCs. These junctions were characterized by microfilament assembly appearing as electron dense structure running the depth of the junction. The phalangeal processes of the outer pillar cells characteristically contained bundles of microtubules (Fig. 4). Ultrastructure of OHCs of group IIA showed distorted shape of their cell bodies with heterochromatic basal nuclei (Figs. 5, 6). In addition, subsurface cisternae appeared rounded or fused forming large vacuoles in the cytoplasm of OHCs (Fig. 6). Wrinkling and irregularity of the lateral plasma membrane were noticed in some OHCs. The Deiters' cells expanded upwards towards the reticular lamina (Fig. 5).
Scanning electron microscopy
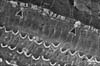
The cochlea of control animals revealed the ordered cellular mosaic pattern made of the apical domains of hair cells and supporting cells in the organ of Corti which formed the reticular lamina. A single row of IHCs were separated from the three rows of OHCs by the apices of the inner pillar cells. Three rows of Deiters' cells were present between the OHCs. Hensen's cells were the outermost large cells positioned further laterally in the organ of Corti (Fig. 7). Each Deiters' cell is formed of the main cell body with its upper part forming a deep seat to enclose the basal portion of OHCs and its innervations. A phalangeal process extends from the Deiters' cell body towards the reticular lamina and terminates with an expansion (the head plate) which contributes to the reticular lamina by filling the spaces between OHCs (Fig. 8). IHCs and OHCs were characterized by tufts of stereocilia protruding from their apices. Stereocilia were cylindrical protrusions morphologically similar to large microvilli with granular appearance of their surface membrane. The tufts of stereocilia were mainly arranged in 3-4 rows with the shortest row seen on the inner aspect of the tuft. The apical tips of shorter stereocilia were connected diagonally to the sides of the adjacent taller stereocilia of the next row by thin filaments called tip links. Stereocilia in the same row were also connected to each other by side links. The stereocilia of IHCs formed a straight or gently curved bundles (Fig. 9), whereas, the OHC stereocilia formed a V-shaped pattern, with the tip of V directed externally (Fig. 10).
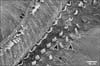
SEM of group IIA revealed that the surface changes of the cochlea were more noticeable in the basal turn of the cochlea. Although both types of hair cells were affected, the complete loss of the surface stereocilia was more prevalent in the OHCs than the IHCs (Figs. 11, 12, 13). Some IHCs revealed disorganization of the tufts of stereocilia and loss of their links (Figs. 11, 12). Stereocilia on the apical surfaces of many OHCs revealed disordering of their regular arrangement and grouping into tufts. Individual stereocilia were bent at their bases and appeared separated from neighboring stereocilia with loss of tip and side links. Other OHCs showed complete loss of stereocilia leaving only a linear group of short stumps remaining on their apices. These changes were more obvious in the outer two rows of OHCs (Figs. 11, 13). Occasionally, complete loss of OHCs was observed with expansion of the apical poles of the surrounding supporting cells, forming "phalangeal scars" (Fig. 13).
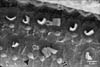
In group IIB, the most characteristic change observed in SEM of the organ of Corti of this group was the frequent detection large blebs with granular surfaces. The blebs appeared as balloon-like protrusions emerging from the cuticular plates of many IHCs and OHCs (Figs. 14, 15, 16, 17, 18). Disarray and loss of the regular arrangement of stereociliary bundles with increased surface granularity of the stereocilia were observed on many IHCs in the IHC row (Figs. 14, 15). In the OHC rows, stereociliary distortion and disarrangement were frequently noticed. Many OHCs revealed degeneration of the tufts of stereocilia with loss of stereociliary bundles. Complete loss of hair cells and expansion of surrounding supporting cells forming phalangeal scars were frequently observed (Figs. 16, 17, 18).
Discussion
The present work demonstrated the deleterious effect of nicotine on the OHCs which revealed distorted shape, heterochromatic nuclei and vacuolated cytoplasm. The apoptotic changes of the nuclei coincide with the result of Berger et al. [16] who reported that activation of nicotinic acetylcholine receptors by low doses of nicotine induces neuronal apoptosis. The OHCs have nicotinic acetylcholine receptors which are activated by the neurotransmitter acetylcholine [17]. The appearance of cytoplasmic vacuoles could be due to dilatation and fusion of subsurface cisternae which would result in wrinkling of the plasma membrane and distortion of the shape of the hair cell body. In support with this suggestion, the subsurface cisternae are supposed to maintain the cylindrical shape of OHC by stiffening its surface [18].
The phalangeal scars that we occasionally observed in topography of nicotine treated cochlea could be correlated with our findings in TEM which revealed the expansion of Deiters' cells upwards towards the reticular lamina. These findings coincide with those reported by previous workers in adult and young mammalian aminoglycoside ototoxicity [19]; in response to hair cell loss [9]. According to these authors the supporting cells (Deiters' and pillar cells) adjacent to the missing hair cells expanded and form these permanent epithelial scars. The developing expansions from Deiters' and pillar cells fill the spaces previously occupied by the missing hair cells and adopt various shapes resulting in the formation of a disordered cellular mosaic appearance over the reticular lamina.
Hensen's cells have also been observed in this work by light and TEM closing in, on the pillar cells. In accordance with our finding, Taylor et al. [20] reported that with the loss of hair cells, the supporting cells are stimulated to change their shape together with an inward migration of cells from the outer edges of the organ of Corti strip. This would make Hensen's cells get closer to the pillar cell region, thus remodeling the structure of organ of Corti after hair cell loss [20].
Nicotine administration induced stereociliary changes as being bent and/or disorganized with a consequent loss of their tip and side links. Stereocilia are membrane bound cellular projections at the apical surface of hair cells that contain a dense core of actin filaments along with actin-associated proteins [21]. It has been known that the V-shape of the rows of stereocilia on the OHCs and the straight-line arrangement on IHCs were both appropriate for a maximal sensitivity of the hair cells to deflection of the stereocilia in a radial direction [22]. The staircase-shaped stereocilia bundles of the hair cells detect mechanical stimuli by the activation of mechano-electrical transduction channels at the stereocilia tips [23]. Also, the tip links and side links between adjacent stereocilia on the hair cell were reported to be important for sensory transduction [22]. Actin depolymerization of the stereocilia was hypothesized to account for hearing loss [24]. The malformation in the sensory hair cells and their stereocilia would consequently lead to clinical auditory impairment as recorded in human [18], in students [25] and in experimental animals as guinea pigs [24].
Topography of the hair cells revealed that nicotine induced hair cell damage in the basal turn of the cochlea of guinea pigs with more affection of the OHCs. The use of high dose of nicotine, for the same period of time, exacerbates the structural changes. In support with our findings, loss and degeneration of OHCs were thought to be the most common form of cochlear damage that underlie most cases of mild to moderate sensory hearing loss [26]. OHCs are responsible for the motion of the basilar membrane through rapid and slow contractions, thus producing cochlear amplification [27]. It has been reported that diffuse damage of up to 30% of OHCs may occur without detectable hearing loss [28]. Smokers were found to have a higher prevalence rate of tinnitus [6], which was thought to be caused by OHC injury [29] whereas severe to profound sensory hearing loss included loss of OHCs and IHCs [30].
The balloon-like protrusions (blebs) of the apical plasma membrane have been observed in this work in relation to IHCs and/or OHCs by SEM. Similar findings have been previously noticed in cisplatin-treated guinea pigs [31] and following loud auditory stimulation [32]. Being noticed in relation to the IHC in an in vitro isolated organ of Corti of adult guinea pigs [33], these authors considered these blebs as physiological features arising as a result of an imbalance of endocytosis and exocytosis in the apical plasma membrane, linked to Na+ loading which occurs in vitro [33]. On the other hand, they have been proposed to be an early degenerative change that develops when actin attachments between the plasma membrane of the hair cell and the underlying cytoskeleton were weakened [31]. Since these blebs were characteristically observed in this work following the use of high dose of nicotine they could be as an association with apoptotic hair cell injury as suggested by [34].
Our work revealed that the deleterious effect of nicotine involved mainly the basal turn of cochlea, which is the region where higher sound frequencies are transduced [35]. This finding could be correlated with the clinical findings that smoking increases the risk for high frequency hearing loss (at 4 kHz) in a dose dependent manner, whereas low frequency hearing loss (at 1 kHz) remained unchanged [36]. In addition, long term smoking with noise exposure was reported to be at a greater risk for developing a permanent hearing loss at high frequencies (3-4 kHz) than nonsmokers [37]. More recently, Paschoal and Azevedo [6] reported that the effects of smoking involved the most basal portion of the cochlea, affecting high sound frequencies permanently and progressively.
In conclusion, nicotine-induced structural damage of sensory hair cells involves mainly the basal cochlear turn and the outer rows in particular. Blebing of the outer plasma membrane of the hair cells occurs as an association with hair loss following the use of high dose of nicotine.




 PDF
PDF ePub
ePub Citation
Citation Print
Print












 XML Download
XML Download