Abstract
Uncontrolled self-renewal plays a direct function in the progression of different types of carcinomas. The same molecular pathway that manages self-renewal in normal stem cells also seems to manage cancer stem cells. Here, we examine the expressions of self-renewal regulatory factors Oct4, Nanog, Sox2, nucleostemin, Zfx, Esrrb, Tcl1, Tbx3, and Dppa4 in tissue samples of colon, prostate, and bladder carcinomas as well as cancer cell lines HT-29, Caco-2, HT-1376, LNCaP, and HepG2. We used reverse transcriptase polymerase chain reaction to examine expressions of the above mentioned regulatory factors in cancer cell lines HT-29, Caco-2, HT-1376, LNCaP, and HepG2 and in 20 tumor tissue samples. Total RNA was isolated by the ISOGEN method. RNA integrity was checked by agarose gel electrophoresis and spectrophotometry. Expressions of Oct4 and nucleostemin at the protein level were determined by immunocytochemistry. A significant relationship was found between tumor grade and self-renewal gene expression. Expressions of stem cell specific marker genes were detected in all examined cancer cell lines, in 40% to 100% of bladder cancer samples, and in 60% to 100% of colon and prostate cancer samples. Oct4 expressed in 100% of tumor tissue samples. Our data show that stem cell markers Oct4, Nanog, Sox2, nucleostemin, Bmi, Zfx, Esrrb, Tcl1, Tbx3, and Dppa4 significantly express in cancer cell lines and cancer tissues. Hence, these markers might be useful as potential tumor markers in the diagnosis and/or prognosis of tumors.
Uncontrolled self-renewal is proposed to be an important mechanism in carcinogenesis [1]. On the basis of the cancer stem cell (CSC) hypothesis, a tumor may be sustained by a subset of cancer cells with stem cell-like features that have the ability for self-renewal and pluripotency [2, 3]. These CSCs have tumorigenic potential and proliferate indistinctly [4]. The same molecular pathway that manages self-renewal in normal stem cells also seems to manage CSCs in tumors [5]. Octamer 3/4 (Oct 3/4), a member of the POU family, is considered to be an important stem cell marker and essential transcription factor during human embryogenesis [6]. In recent years, there have also been reports about the presence of Oct 3/4 in differentiated benign and malignant human cells [7]. Sox2 is a member of the Sox (SRY-Related HMG box) gene family that encodes transcription factors with a single HMG DNA-binding domain. Sox2 controls neural progenitor cells by prohibiting their ability to differentiate [8]. Sox2 is also expressed in several malignant tissues [9]. Nanog is believed to function in conjunction with other factors such as Oct4 and Sox2 to form an embryonic stem cell identity [10, 11]. Recently, Nanog expression has been reported in human neoplasms, including germ cell tumors, breast carcinoma, and osteosarcoma [12]. Nucleostemin is expressed in the nucleoli of adult central nervous system stem cells, primitive bone marrow cells, embryonic stem cells, and several cancer cell lines [13]. Nucleostemin is often used as a stem cell marker [14]. Bmi is a potent repressor of retinoblastoma and the p53 pathway, its role in tumorigenesis is very important [15]. It is a transcriptional repressor that belongs to the polycomb-group family of proteins that are involved in hematopoiesis, regulation of proliferation, and senescence [16]. The Bmi gene is widely expressed in diverse human tumors including lymphomas, bladder and breast carcinomas, and neuroblastoma [17]. Zfx is a zinc finger protein of the Zfy family. Zfx normally suppresses apoptosis in embryonic stem cells. Zfx directly activates common target genes in embryonic and hematopoietic stem cells, as well as embryonic stem cell-specific target genes such as embryonic stem cell self-renewal regulation of Tbx3 and Tcl1 [18]. The Tbx3 gene shares a common DNA binding domain. The T-box genes encode transcription factors involved in regulation of the developmental process [19, 20]. Tcl1 has been extensively studied as an oncogene in T-cell leukemia and plays an important role in early mouse embryos and embryonic stem cells [21]. Esrrb in coordination with Nanog and Oct4 activate the internal machinery of ES cells [22]. The Dppa4 protein is located in the embryonic stem cell nucleus and is associated with chromatin. It regulates the differentiation of ES cells into a primitive ectoderm lineage [23]. Although the expression of these genes in embryonic stem cells has been extensively studied, little is known about their expressions in somatic cancers. Colon, prostate, bladder, and hepatocarcinomas are common worldwide carcinomas, whose diagnoses and treatments are challenging [24, 25, 26]. We have examined the expressions of Oct4, Sox2, Nanog, nucleostemin, Bmi, Zfx, Tcl1, Tbx3, Dppa4, and Esrrb these genes in various cancers. It is possible that these genes participate in the development and regulation of tumor and hyperplastic tissues. Thus, we have used a number of malignant tumor tissues and cancer cell lines to investigate the expressions of these genes with the intent to study the mechanism of cancer cell self-renewal.
Prostate, bladder, and colon cancer samples were obtained from patients who referred to Tohid Hospital, Sanandaj and Imam Khomeini Hospital, Tehran, Iran. Normal tissue samples (apparently normal tissues from the margins of colon cancer samples) were collected. Samples were immediately snap-frozen in liquid nitrogen and stored at -70℃ until used for RNA extraction. All patients consented to have their biopsy samples analyzed. This study was approved by the Ethics Committees of Kurdistan University of Medical Sciences.
LNCaP (human prostate cancer), HepG2 (hepatocarcinoma), HT-1376 (bladder cancer), U87 (glioblastoma), NT2 (embryonal carcinoma stem cell), HT-29, and Caco-2 (colon cancers) were maintained at 37℃ in a humidified atmosphere of 5% CO2 with RPMI1640 medium (Gibco, Paisley, UK), supplemented with 100 U/ml penicillin, 100 µg/ml streptomycin, and 10% fetal bovine serum (FBS; Gibco). Caco-2 and NT2 were cultured in Dulbecco's modified Eagle medium supplemented with 15% FBS. Passaging was routinely performed with 0.25% Trypsin/EDTA.
Total RNA was isolated from frozen tissues and cell lines using the RNX-Plus solution (CinnaGen, Tehran, Iran) according to the manufacturer's instructions. Purity and integrity of the extracted RNA was measured by UV spectrophotometry (260/280 nm ratio) as well as visual observation of samples by agarose gel electrophoresis. Total RNA was treated with RNase-free DNase (Fermentas, St. Leon-Rot, Germany) for which 1 µg was used for cDNA synthesis, with the RT Kit (reverse transcriptase polymerase chain reaction [RT-PCR] Kit, Bioneer, Daejeon, Korea) and random hexamer primer in a 20 µl reaction according to the manufacturer's instructions. The RT-PCR Kit consisted of 5× buffer, dNTP, RNAsin and RT enzyme (RevertAid M-MuLV reverse transcriptase). For each sample, a non-RT control was used in parallel to detect any potential nonspecific ampli fication of contaminated genomic DNA. PCR primers were designed by Gene Runner and Prime 3 software. The primer sequences of the genes are shown in Table 1. PCR was conducted with 2 µl of cDNA sample, 0.2 µl of Taq polymerase (CinnaGen), 0.75 µl MgCl2 (25 mM), 0.5 µl dNTP (10 mM), 2.5 µl 10× buffer, 0.5 µl of each primer (20 mM), and 18 µl dH2O. We used glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as an internal standard for RT-PCR. PCR amplification was performed for either 36 (Oct4, Nanog, Sox2, Bmi), 40 (nucleostemin, Tcl1, Esrrb, Dppa4), or 32 (Tbx3, Zfx) cycles with the following cycle conditions: 94℃ for 45 seconds, 60℃ (Oct4, Nanog, Sox2, nucleostemin); 59℃ (GAPDH); 58℃ (Tbx3); and 62℃ (Dppa4, Esrrb) for 45 seconds by the Thermal cycler (Eppendorf, Hamburg, Germany). PCR products were separated on a 1% agarose gel, stained with ethidium bromide and then visualized and photographed with a UV trans-illuminator (UVIdoc, Cambridge, UK).
The expressions of Oct4 and nucleostemin at the protein level were determined by immunostaining in the LNCaP, HepG2, and HT-1376 cell lines.
Briefly, different cell cultures were plated in 4-well dishes until confluent. Next, cells were fixed with 4% paraformaldehyde for 15 minutes at room temperature. After washing with phosphate buffered saline (PBS), fixed cells were permeablized with 0.25% Triton X-100. The cells were then incubated in the appropriate diluted (1:50) first anti bodies (affinity-purified goat polyclonal anti-Oct4 for Oct4; and anti-nucleostemin for nucleostemin; Santa Cruz Biotechnology) in PBS that contained 1% bovine serum albumin (BSA) for 1 hour at room temperature. After washing for three times with PBS, cells were incubated with the appropriate secondary antibodies (Texas red-conjugated donkey anti-goat for Oct 4 or FITC-conjugated donkey anti-goat for nucleostemin, Santa Cruz Biotechnology, Santa Cruz, CA, USA) at a dilution of 1:100 in 1% BSA for 1 hour at room temperature. Cells were then washed three times with PBS. For the negative control all conditions were kept the same, except that the primary antibody was omitted. Fluorescent images were obtained with a Nikon TE-200 fluorescent microscope equipped with a digital camera (Nikon, Tokyo, Japan).
Stem cell markers were expressed in malignant human colon, prostate and bladder tissues as well as the cancer cell lines (HT-29, Caco-2, HT-1376, LNCaP, and HepG2). Specific primers were used to amplify segments of the Oct4, Nanog, Sox2, nucleostemin, Bmi, Zfx, Esrrb, Dppa4, Tcl1, and Tbx3 genes and examined their expressions by RT-PCR (Table 1). As expected, DNA fragments of these genes, based on the designated primers (Table 1), were amplified in the PCR reactions.
Specific primers for Oct4 have been designed [27] to amplify a particular segment of Oct4 that is common in both spliced variants of the gene (GenBank accession numbers: NM_002701 and NM_203289). We detected the expression of Oct4 in all (100%) tumor samples (colon, bladder, and prostate) as well as the hepatic cancer cell lines (Tables 2, 3, Figs. 1, 2, 3, 4, 5, 6). Nanog expression was detected in 100% of the colon cancer samples, 90% of the bladder cancer samples, and 80% of the prostate cancer samples. Nucleostemin was detected in 100% of the prostate cancer samples, 80% of the bladder cancer samples, and 60% of the colon cancer samples (Tables 2, 3, Figs. 1, 2, 3, 4, 5, 6).
As seen in Figs. 1, 2, 3, 4, 5, 6, 7 and Table 2, expressions of all ten genes were detected in 40% to 100% of the bladder cancer tissue, and 60% to 100% of both colon and prostate tumor samples.
We also checked for expression of the ten genes in five normal colon tissues. Only low-level expressions of Oct4 and NS genes were detected (Fig. 5).
There was a significant relation between tumor grade and expression of self-renewal genes. In the prostate cancer samples, 60% of the self-renewal genes were expressed in grade I, 80% in grade II, and 90% in grade III tumors. In colon cancer samples, 40% to 100% of the self-renewal genes were expressed in grade I, 70% in grade II, and 60% to 80% in grade III tumors. Bladder cancer samples expressed about 60% of the self-renewal genes in grade I tumors, 70%, in grade II tumors, and 100% in grade III tumors. Expression of Oct4 was detected in all tumor grades of the prostate, colon, and bladder cancer samples. Expressions of Oct4, Nanog, Sox2, NS, Zfx, Tbx3, Tcl1, and Esrrb genes were detected in 100% of the grade III cancer samples from prostate, colon, and bladder tissues (Table 3, Fig. 6).
We used the polyclonal anti-Oct4 and anti-nucleostemin antibody to examine subcellular localizations of Oct4 and Nucleostemin proteins in the cancer cell lines by immunocytochemistry (ICC). NT2, a human embryonic carcinoma cell line, was the positive control, which showed the same pat tern of antigen expression as previously described [28]. U87, a human glioblastoma cell line, was used as the positive control to optimize the experiment for NS, which has nucleolar localization for nucleostemin. Our results confirmed expression of Oct4 (Fig. 7) and nucleostemin (Fig. 8) in the cancer cell lines. There was no staining signal in the negative controls, where all conditions were the same with the exception of the primary antibodies. The experiment was repeated twice, with similar results.
A specific aim in cancer research is to determine the mecha nism by which CSCs arise and self-renew [1, 2, 29]. The CSC hypothesis proposes that only a small degree of tumor cells are enriched for cancer initiation, causing tu mor development [1, 2, 3]. This new idea of cancer may also alter our diagnostic and therapeutic goals by allowing us to better determine and target CSCs [5, 6]. Uncontrolled self-renewal plays a direct function in different types of carcinoma progression, or profits as a precious marker of tumorigenesis [4, 5, 29].
In this study, we have examined the expressions of self-renewal regulatory factors such as Oct4, Nanog, Sox2, nucleostemin, Bmi, Zfx, Esrrb, Tcl1, Tbx3, and Dppa4 in different cancer types and cancer cell lines. These genes are required for efficient self-renewal of embryonic stem cells [12, 13, 14, 15, 16, 17].
In many germ cell tumors as well as a few somatic tumors, there is observable expression of Oct4 [9, 30, 31]. Oct4 can function as a potent oncogene in vivo and support the image that progenitor cells are an energetic force in tumorigenesis [32]. Oct4 expression has been detected in human breast, liver, pancreas, colon, kidney, and gastric cancers, as well as in Hela and MCF7 cells, and bladder tumors [33, 34, 35, 36, 37, 38].
In this study, we detected the expression of Oct4 in all cancer cell lines. ICC analysis further determined the expression of Oct4 at the protein level. Looijenga et al. [35] have previously reported a lack of expression of Oct4 in a panel of somatic tissues such as bladder tumors [35], this finding does not agree with our study conclusion. Possibly, this inconsistency might be due to the heterogeneous nature of tumors [36]. Recently, several groups have reported the expression of Oct4 protein in several somatic tissues [37, 38]. In 2007, Atlasi et al. [33] have reported expression of Oct4 in bladder tumors, which confirmed our results. In our study, Nanog expression was detected in 100% of the colon cancer samples, 90% of the bladder cancer samples and 80% of the prostate cancer samples. Recently, Nanog expression has been reported in human neoplasms, including germ cell tumors, breast carcinoma and osteosarcoma [12, 13]. Ezeh et al. [12], in 2005, have reported elevated expression levels of Oct4 together with Nanog, Stellar, and Gdf3 mRNA in both breast carcinoma tissue and breast carcinoma cell line MCF7. These results have suggested that breast carcinomas, and possibly other human malignancies, may contain cells remnant of embryonic-like stem cells [11, 12]. In addition, a clinical survey showed that elevated expression of Nanog was related to retinoblastoma, prostate cancer, embryonal carcinoma, metastatic germ cell tumor, and ovarian cancer [38]. Taken together, these findings have indicated that abnormal expressions of Oct4 and Nanog in stem cell and tumor tissues might play a vital role in tumor transformation, tumorigenicity, and tumor metastasis [39, 40]. Hoei-Hansen et al. [38] has stated that Sox2 is overexpressed in patients with ovarian carcinoma. In the present study, we have found the expressions of Oct4, Nanog, and Sox2 in several human tumors. In addition, we observed low expression of Oct4 in some normal colon tissues that were obtained from asymptomatic individuals. Recently, Tai et al. [7] have shown that Oct4 is expressed in several adult human stem cells and Matthai et al. [37] have reported Oct4 expression in normal human endometrium. The observed expression of Oct4 in normal colon tissues might reflect the presence of rare normal colon stem cells in these samples.
Nucleostemin expressed in human placental tissues also at a high level, possibly due to the existence of a mass of placental stem cells [40, 41]. In the present study, nucleostemin highly expressed in cancer tissues and cancer cell lines. We detected poor expression of nucleostemin in some normal colon tissues. Recently, a small quantity of nucleostemin expression has been detected in normal human muscle [42, 43], possibly in few myoblasts with stem cell characteristics existing in muscle tissues [43]. ICC demonstrated that LNCaP, HepG2, and HT-1376 cancer cell lines showed cellular staining for nucleostemin proteins. These results were similar to those of previous studies of other human cancers. In normal stem cells and U87-glioblastoma cell line, nucleostemin protein has been shown to localize in the nucleolus [13, 44]. However, cancer cell lines that we used exhibited both cytoplasmic and nucleolar localizations of nucleostemin. This distribution pattern of nucleostemin has recently been observed in gastric, prostate, and renal cell carcinomas [44, 45]. Recently, a novel nucleostemin binding protein was identified as an alpha isoform of human protein phosphatase 2 regulatory subunit B (B56 PPP2R5A). PPP2R5A is located in the cytoplasm [46], which may explain to some extent why nucleostemin protein displays both cytoplasmic and nucleolar locations; however, further investigations are necessary to determine why it occurs at this exact location.
In this study, Bmi overexpressed in human bladder, colon and prostate cancers as well as cancer cell lines. The results of our research were consistent with previous reports from other cancers. The Bmi gene is widely expressed in diverse human tumors such as lymphoma, bladder cancer, breast cancer, and neuroblastoma [16, 17, 18]. There are numerous reports on the expressions of Zfx, Tcl1, Tbx3, Esrrb, and Dppa4 in cancers. In this study, we have aimed to explore the expressions of these genes in human cancers. RT-PCR analysis confirmed expressions of Zfx, Tcl1, and Tbx3 in human bladder, colon and prostate cancers as well as cancer cell lines. Zfx is a transcription factor specifically required in ESC embryonic and adult stem cell populations such as hematopoietic stem cells. In addition to common target genes [20], the Zfx gene is highly expressed in glioma tissue and cell lines [47]. In addition, it can directly activate cell type-specific targets such as Tbx3 and Tcl1 in embryonic embryonic stem cells [21]. The pattern of Tcl1 expression as well as its defects from hematopoietic stem cells indicates its potential to act as a highly specific drug target in specific malignancies of lymphoid and germ-cell origin [22]. Lau et al. [48], in 2010, have shown protein expression of Tcl1 in testicular germ cell tumors. Overexpression of Tbx3 has been described in breast, liver, and ovarian cancers in addition to endometrioid adenocarcinomas [49, 50, 51]. Based on the key role of Tbx3 in carcinogenesis, inhibition of Tbx3 activity could be an effective therapeutic strategy for human cancer [52]. Esrrb has been reported to interplay with the ES cell embryonic stem cell master regulator Nanog. Many of these potential Esrrb target genes comprise Oct4, Sox2, and Nanog-regulated genes and include the respective promoters of these core ES cell embryonic stem cellregulators [53]. Expression of the Dppa4 gene in embryonic stem cells has been studied, but little is known about its expression in somatic cancer [23]. There is no published report on the expression of these genes in cancers. We reported the expressions of Dppa4, Esrrb, Tcl1, Tbx3, and Zfx in human bladder, colon and prostate cancers as well as cancer cell lines. The results of our research confirmed previous reports of other cancers.
In conclusion, our data is a report on the expression of the ES cell marker Nanog, nucleostemin, Sox2, Zfx, Tcl1, Tbx3, Esrrb, and Dppa4 in bladder, prostate, and colon cancers as well as in cancer cell lines. Therefore, this data might provide valuable information on the nature and behavior of tumors, leading to a new strategy for targeting CSCs and perhaps one step closer to the prevention of cancer recurrence and metastasis. However, further studies are required to isolate and characterize the putative CSCs from tumors and elucidate the role of gene self-renewal in tumor carcinogenesis.
Figures and Tables
Fig. 1
Reverse transcriptase polymerase chain reaction (RT-PCR) analysis of the expressions of Oct4, Nanog, Sox2, nucleostemin, Esrrb, Tbx3, Tcl1, Dppa4, Zfx, and Bmi in HT-1376 (bladder cancer cell line), LNCaP (pro state cancer cell line), HepG2 (hepa tocarcinoma cell line), HT-29, and Caco-2 (colon cancer cell lines). RT-PCR analysis was performed using carefully designated primers for specific amplification of each gene. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was the internal control. U87, a glioblastoma cell line, was the positive control for Sox2, nucle ostemin, and Zfx. NT2, a human embryonal carcinoma cell line, was the positive control for Oct4, Nanog, Bmi, Tcl1, Tbx3, Dppa4, Zfx, and Esrrb. Note: All genes were expressed in the cancer cell lines.

Fig. 2
Reverse transcriptase polymerase chain reaction analysis shows the expressions of Oct4, Nanog, Sox2, nucleostemin, Esrrb, Tbx3, Tcl1, Dppa4, Zfx, and Bmi in bladder cancer tissues. Note: Some samples did not express some of genes. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the internal control.
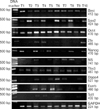
Fig. 3
Reverse transcriptase polymerase chain reaction analysis shows expressions of Oct4, Nanog, Sox2, nucleostemin, Esrrb, Tbx3, Tcl1, Dppa4, Zfx, and Bmi in prostate cancer tissues. Although all genes were expressed in some of the samples, some samples did not express some of genes. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal control.

Fig. 4
Reverse transcriptase polymerase chain reaction (RT-PCR) analysis shows the expressions of Oct4, Nanog, Sox2, nucleostemin, Esrrb, Tbx3, Tcl1, Dppa4, Zfx, and Bmi in the colon cancer tissues. RT-PCR analysis was performed using carefully designated primers for specific amplification of each gene. Note: All genes were expressed in some of the samples, however other samples did not express some of genes. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal control.
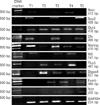
Fig. 5
Reverse transcriptase polymerase chain reaction analysis shows expression of Oct4, Sox2, Nanog, Tcl1, Tbx3, Esrrb, Dppa4, and nucleostemin in normal colon tissues. Low expressions of Oct4 and nucleostemin were observed in some normal colon tissues.

Fig. 6
Histogram of polymerase chain reaction results in colon, bladder and prostate cancer samples, as well as HT-29, Caco-2, HT-1376, LNCaP, and HepG2 cancer cell lines. Oct4 was expressed in 100% of bladder, colon, and prostate tumor samples. Expression of Nanog was detected in 100% of the colon cancer samples, 90% of the bladder cancer samples and 80% of the prostate cancer samples. Nucleostemin was detected in 100% of the prostate cancer samples, 80% of the bladder cancer samples, and 60% of the colon cancer samples.

Fig. 7
Immunocytochemistry (ICC) of cancer cell lines stained with anti-Oct4 antibody. (A) NT2 cells were used as positive controls. HT-1376 (C), HepG2 (E), and LNCaP (G) cell lines were positively stained for Oct4. Panels (B), (D), (F), and (H) show the phase contrast counterparts of panels (A), (C), (E), and (G), respectively. Result is representative of three independent experiments. Scale bars=50 µm (A-H).

Fig. 8
Immunocytochemistry (ICC) of the cancer cell lines stained with anti-nucleostemin antibody. (A) NT2 cells were used as positive controls. HT-1376 (C), HepG2 (E), and LNCaP (G) cell lines were positively stained for nucleostemin. Panels (B), (D), (F), and (H) show the phase contrast counterparts of panels (A), (C), (E), and (G), respectively. Result is representative of three independent experiments. Scale bar=50 µm (A-H).
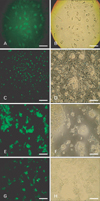
Table 3
Table shows relations between grade of cancer tissue samples and expressions of self-renewal genes

*Columns contain positive results (80% to 90%) of self-renewal gene expression for different genes in bladder, prostate and colon cancer tissues. †Columns contain positive results (100%) of self-renewal gene expression for different genes in bladder or prostate cancer samples. ‡Columns show positive results (90% to 100%) of self-renewal gene expression for same genes in all three types of cancer tissues.
References
1. Cho RW, Clarke MF. Recent advances in cancer stem cells. Curr Opin Genet Dev. 2008; 18:48–53.
2. Clarke MF, Fuller M. Stem cells and cancer: two faces of eve. Cell. 2006; 124:1111–1115.
3. Al-Hajj M, Clarke MF. Self-renewal and solid tumor stem cells. Oncogene. 2004; 23:7274–7282.
4. Klonisch T, Wiechec E, Hombach-Klonisch S, Ande SR, Wesselborg S, Schulze-Osthoff K, Los M. Cancer stem cell markers in common cancers: therapeutic implications. Trends Mol Med. 2008; 14:450–460.
5. Pardal R, Clarke MF, Morrison SJ. Applying the principles of stem-cell biology to cancer. Nat Rev Cancer. 2003; 3:895–902.
6. Lee J, Kim HK, Rho JY, Han YM, Kim J. The human OCT-4 isoforms differ in their ability to confer self-renewal. J Biol Chem. 2006; 281:33554–33565.
7. Tai MH, Chang CC, Kiupel M, Webster JD, Olson LK, Trosko JE. Oct4 expression in adult human stem cells: evidence in support of the stem cell theory of carcinogenesis. Carcinogenesis. 2005; 26:495–502.
8. Bani-Yaghoub M, Tremblay RG, Lei JX, Zhang D, Zurakowski B, Sandhu JK, Smith B, Ribecco-Lutkiewicz M, Kennedy J, Walker PR, Sikorska M. Role of Sox2 in the development of the mouse neocortex. Dev Biol. 2006; 295:52–66.
9. Schoenhals M, Kassambara A, De Vos J, Hose D, Moreaux J, Klein B. Embryonic stem cell markers expression in cancers. Biochem Biophys Res Commun. 2009; 383:157–162.
10. Pan G, Thomson JA. Nanog and transcriptional networks in embryonic stem cell pluripotency. Cell Res. 2007; 17:42–49.
11. Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, Smith A. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003; 113:643–655.
12. Ezeh UI, Turek PJ, Reijo RA, Clark AT. Human embryonic stem cell genes OCT4, NANOG, STELLAR, and GDF3 are expressed in both seminoma and breast carcinoma. Cancer. 2005; 104:2255–2265.
13. Fan Y, Liu Z, Zhao S, Lou F, Nilsson S, Ekman P, Xu D, Fang X. Nucleostemin mRNA is expressed in both normal and malignant renal tissues. Br J Cancer. 2006; 94:1658–1662.
14. Kafienah W, Mistry S, Williams C, Hollander AP. Nucleostemin is a marker of proliferating stromal stem cells in adult human bone marrow. Stem Cells. 2006; 24:1113–1120.
15. Lessard J, Sauvageau G. Bmi-1 determines the proliferative capacity of normal and leukaemic stem cells. Nature. 2003; 423:255–260.
16. Leung C, Lingbeek M, Shakhova O, Liu J, Tanger E, Saremaslani P, Van Lohuizen M, Marino S. Bmi1 is essential for cerebellar development and is overexpressed in human medulloblastomas. Nature. 2004; 428:337–341.
17. Qin ZK, Yang JA, Ye YL, Zhang X, Xu LH, Zhou FJ, Han H, Liu ZW, Song LB, Zeng MS. Expression of Bmi-1 is a prognostic marker in bladder cancer. BMC Cancer. 2009; 9:61.
18. Cellot S, Sauvageau G. Zfx: at the crossroads of survival and self-renewal. Cell. 2007; 129:239–241.
19. Galan-Caridad JM, Harel S, Arenzana TL, Hou ZE, Doetsch FK, Mirny LA, Reizis B. Zfx controls the self-renewal of embryonic and hematopoietic stem cells. Cell. 2007; 129:345–357.
20. Ivanova N, Dobrin R, Lu R, Kotenko I, Levorse J, DeCoste C, Schafer X, Lun Y, Lemischka IR. Dissecting self-renewal in stem cells with RNA interference. Nature. 2006; 442:533–538.
21. Lock RB. TCL1: a new drug target in lymphoid and germ-cell malignancies? Int J Biochem Cell Biol. 2003; 35:1614–1618.
22. Zhang X, Zhang J, Wang T, Esteban MA, Pei D. Esrrb activates Oct4 transcription and sustains self-renewal and pluripotency in embryonic stem cells. J Biol Chem. 2008; 283:35825–35833.
23. Chakravarthy H, Boer B, Desler M, Mallanna SK, McKeithan TW, Rizzino A. Identification of DPPA4 and other genes as putative Sox2:Oct-3/4 target genes using a combination of in silico analysis and transcription-based assays. J Cell Physiol. 2008; 216:651–662.
24. Tejpar S. The multidisciplinary management of gastrointestinal cancer. The use of molecular markers in the diagnosis and treatment of colorectal cancer. Best Pract Res Clin Gastroenterol. 2007; 21:1071–1087.
25. Cohen SM, Shirai T, Steineck G. Epidemiology and etiology of premalignant and malignant urothelial changes. Scand J Urol Nephrol Suppl. 2000; (205):105–115.
26. Parkin DM, Pisani P, Ferlay J. Global cancer statistics. CA Cancer J Clin. 1999; 49:33–64.
27. Kermani AJ, Fathi F, Mowla SJ. Characterization and genetic manipulation of human umbilical cord vein mesenchymal stem cells: potential application in cell-based gene therapy. Rejuvenation Res. 2008; 11:379–386.
28. Matin MM, Walsh JR, Gokhale PJ, Draper JS, Bahrami AR, Morton I, Moore HD, Andrews PW. Specific knockdown of Oct4 and beta2-microglobulin expression by RNA interference in human embryonic stem cells and embryonic carcinoma cells. Stem Cells. 2004; 22:659–668.
29. Moserle L, Ghisi M, Amadori A, Indraccolo S. Side population and cancer stem cells: therapeutic implications. Cancer Lett. 2010; 288:1–9.
30. Schulz WA, Hoffmann MJ. Transcription factor networks in embryonic stem cells and testicular cancer and the definition of epigenetics. Epigenetics. 2007; 2:37–42.
31. Santagata S, Ligon KL, Hornick JL. Embryonic stem cell transcription factor signatures in the diagnosis of primary and metastatic germ cell tumors. Am J Surg Pathol. 2007; 31:836–845.
32. Hochedlinger K, Yamada Y, Beard C, Jaenisch R. Ectopic expression of Oct-4 blocks progenitor-cell differentiation and causes dysplasia in epithelial tissues. Cell. 2005; 121:465–477.
33. Atlasi Y, Mowla SJ, Ziaee SA, Bahrami AR. OCT-4, an embryonic stem cell marker, is highly expressed in bladder cancer. Int J Cancer. 2007; 120:1598–1602.
34. Jin T, Branch DR, Zhang X, Qi S, Youngson B, Goss PE. Examination of POU homeobox gene expression in human breast cancer cells. Int J Cancer. 1999; 81:104–112.
35. Looijenga LH, Stoop H, de Leeuw HP, de Gouveia Brazao CA, Gillis AJ, van Roozendaal KE, van Zoelen EJ, Weber RF, Wolffenbuttel KP, van Dekken H, Honecker F, Bokemeyer C, Perlman EJ, Schneider DT, Kononen J, Sauter G, Oosterhuis JW. POU5F1 (OCT3/4) identifies cells with pluripotent potential in human germ cell tumors. Cancer Res. 2003; 63:2244–2250.
36. Amini S, Fathi F, Parivar K, Kuchesfahani HM, Rezaie MJ, Nikkhoo B. Evaluating the expression of Oct4, NANOG, Sox2 and nucleostemin in colon cancer cell lines (Caco-2 and HT-29). Yakhteh Med J. 2010; 12:223–230.
37. Matthai C, Horvat R, Noe M, Nagele F, Radjabi A, van Trotsenburg M, Huber J, Kolbus A. Oct-4 expression in human endometrium. Mol Hum Reprod. 2006; 12:7–10.
38. Hoei-Hansen CE, Kraggerud SM, Abeler VM, Kaern J, Rajpert-De Meyts E, Lothe RA. Ovarian dysgerminomas are characterised by frequent KIT mutations and abundant expression of pluripotency markers. Mol Cancer. 2007; 6:12.
39. Cheng L, Sung MT, Cossu-Rocca P, Jones TD, MacLennan GT, De Jong J, Lopez-Beltran A, Montironi R, Looijenga LH. OCT4: biological functions and clinical applications as a marker of germ cell neoplasia. J Pathol. 2007; 211:1–9.
40. Tsai RY, McKay RD. A nucleolar mechanism controlling cell proliferation in stem cells and cancer cells. Genes Dev. 2002; 16:2991–3003.
41. Tsai RY, McKay RD. A multistep, GTP-driven mechanism controlling the dynamic cycling of nucleostemin. J Cell Biol. 2005; 168:179–184.
42. Chen JC, Goldhamer DJ. Skeletal muscle stem cells. Reprod Biol Endocrinol. 2003; 1:101.
43. Berendse M, Grounds MD, Lloyd CM. Myoblast structure affects subsequent skeletal myotube morphology and sarcomere assembly. Exp Cell Res. 2003; 291:435–450.
44. Liu SJ, Cai ZW, Liu YJ, Dong MY, Sun LQ, Hu GF, Wei YY, Lao WD. Role of nucleostemin in growth regulation of gastric cancer, liver cancer and other malignancies. World J Gastroenterol. 2004; 10:1246–1249.
45. Liu RL, Zhang ZH, Zhao WM, Wang M, Qi SY, Li J, Zhang Y, Li SZ, Xu Y. Expression of nucleostemin in prostate cancer and its effect on the proliferation of PC-3 cells. Chin Med J (Engl). 2008; 121:299–304.
46. Yang HX, Jin GL, Meng L, Zhang JZ, Liu WB, Shou CC. Screening and identification of proteins interacting with nucleostemin. World J Gastroenterol. 2005; 11:4812–4814.
47. Zhou Y, Su Z, Huang Y, Sun T, Chen S, Wu T, Chen G, Xie X, Li B, Du Z. The Zfx gene is expressed in human gliomas and is important in the proliferation and apoptosis of the human malignant glioma cell line U251. J Exp Clin Cancer Res. 2011; 30:114.
48. Lau SK, Weiss LM, Chu PG. TCL1 protein expression in testicular germ cell tumors. Am J Clin Pathol. 2010; 133:762–766.
49. Ito A, Asamoto M, Hokaiwado N, Takahashi S, Shirai T. Tbx3 expression is related to apoptosis and cell proliferation in rat bladder both hyperplastic epithelial cells and carcinoma cells. Cancer Lett. 2005; 219:105–112.
50. Yarosh W, Barrientos T, Esmailpour T, Lin L, Carpenter PM, Osann K, Anton-Culver H, Huang T. TBX3 is overexpressed in breast cancer and represses p14 ARF by interacting with histone deacetylases. Cancer Res. 2008; 68:693–699.
51. Renard CA, Labalette C, Armengol C, Cougot D, Wei Y, Cairo S, Pineau P, Neuveut C, de Reyniès A, Dejean A, Perret C, Buendia MA. Tbx3 is a downstream target of the Wnt/betacate nin pathway and a critical mediator of beta-catenin survival functions in liver cancer. Cancer Res. 2007; 67:901–910.
52. Lomnytska M, Dubrovska A, Hellman U, Volodko N, Souchelnytskyi S. Increased expression of cSHMT, Tbx3 and utrophin in plasma of ovarian and breast cancer patients. Int J Cancer. 2006; 118:412–421.
53. van den Berg DL, Zhang W, Yates A, Engelen E, Takacs K, Bezstarosti K, Demmers J, Chambers I, Poot RA. Estrogen-related receptor beta interacts with Oct4 to positively regulate Nanog gene expression. Mol Cell Biol. 2008; 28:5986–5995.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download