Abstract
There is little or no information about the distribution of elastic fibers in the human fetal head. We examined this issue in 15 late-stage fetuses (crown-rump length, 220-320 mm) using aldehyde-fuchsin and elastica-Masson staining, and we used the arterial wall elastic laminae and external ear cartilages as positive staining controls. The posterior pharyngeal wall, as well as the ligaments connecting the laryngeal cartilages, contained abundant elastic fibers. In contrast with the sphenomandibular ligament and the temporomandibular joint disk, in which elastic fibers were partly present, the discomalleolar ligament and the fascial structures around the pterygoid muscles did not have any elastic fibers. In addition, the posterior marginal fascia of the prestyloid space did contain such fibers. Notably, in the middle ear, elastic fibers accumulated along the tendons of the tensor tympani and stapedius muscles and in the joint capsules of the ear ossicle articulations. Elastic fibers were not seen in any other muscle tendons or vertebral facet capsules in the head and neck. Despite being composed of smooth muscle, the orbitalis muscle did not contain any elastic fibers. The elastic fibers in the sphenomandibular ligament seemed to correspond to an intermediate step of development between Meckel's cartilage and the final ligament. Overall, there seemed to be a mini-version of elastic fiber distribution compared to that in adults and a different specific developmental pattern of connective tissues. The latter morphology might be a result of an adaptation to hypoxic conditions during development.
Although elastic fibers are a well-known component of human fibrous tissues, their distribution in human fetuses has not been examined extensively, possibly because of the identification of these fibers is difficult. In the adult human body, elastic fibers generally coexist with smooth muscles, and both show a specific organizational relationship [1-3]. If fetal elastic fibers also coexist with smooth muscles in the head region, then their distribution would be very limited, for example, their distribution in arterial walls and some cartilages, because smooth muscle is fairly scant in the fetal head. We are aware of limited examples of smooth muscle that are not closely related to vessels in the fetal head: the orbitalis muscle behind the orbit [4] and the ciliary muscle. However, elastic fibers can be present without associated smooth muscle in specific structures such as elastic cartilages, the nuchal ligament [5], the yellow ligament (ligamentum flavum) [6, 7], the skin dermis [8], and the facet capsule of the vertebral column [9]. In the last case, Shiraishi et al. [9] used human fetal material for their research. Likewise, specific fascial structures in the human pelvic floor contain elastic fibers without any association with smooth muscle [10-13]. In contrast, information about elastic fibers in the head connective tissue is limited: only a few reported studies of experimental animals [14-16] and humans [17] have demonstrated that the temporomandibular joint contains elastic fibers.
A notable constituent of normal elastic fibers is osteopontin, an acidic matrix protein that is expressed mainly in mineralized tissues [18]; therefore, elastic fibers facilitate calcification [19]. In fact, in the ligamentum flavum, changes in elastic fiber configuration have been well described in relation to calcification [6, 7]. We have noted that Meckel's and Reichert's cartilages in fetal heads undergo significant changes in morphology to form the final derivatives [20-25]. Calcification can occur in Meckel's and Reichert's cartilages at specific sites such as ear ossicles. Therefore, we hypothesized that these cartilages are likely to accompany elastic fibers as an intermediate developmental step before attaining their final morphology. Consequently, the aim of this study was to examine the distribution of elastic fibers in late-stage human fetuses. To identify the elastic fibers, we employed both aldehyde-fuchsin and elastica-Masson staining.
The study was performed in accordance with the provisions of the Declaration of Helsinki 1995 (as revised in Edinburgh 2000). We examined paraffin-embedded sagittal sections of 15 late-stage fetuses (28-37 weeks of gestation; crown-rump length, 220-320 mm). All the specimens were part of a large collection kept at the Embryology Institute of the Universidad Complutense, Madrid, and were the products of urgent abortion, miscarriages, or ectopic pregnancies that were managed at the Department of Obstetrics of the University. The donated fetuses were fixed and stored for >3 months in 10% v/v formalin solution. After trimming the tissue mass, decalcification was performed for 2-5 days at room temperature using Plank-Rychlo solution (7% [w/v] AlCl2 · 6H2O, 3.6% HCl, 4.6% HCOOH; WAKO, Tokyo, Japan). After routine procedures for paraffin-embedded histology, 10-µm thick sections were prepared at intervals of 0.5-1 mm. To identify elastic fibers, we chose 2 different types of staining: 1) aldehyde-fuchsin staining [26, 27] and 2) elastica-Masson staining, a variation of Masson-Goldner staining [28-30]. The aldehyde-fuchsin staining was performed after overnight post-fixation of the section on the glass slide with Bouin's fixative. Because of the severe conditions used for decalcification, immunohistochemistry was not possible with these specimens (data not shown).
As positive controls for aldehyde-fuchsin and elastica-Masson staining, we used the elastic laminae of the arterial walls, nuchal skin, and elastic cartilage of the external auditory meatus (Fig. 1). The nuchal ligament had not yet developed in these specimens. The elastic laminae of the external carotid artery were selectively stained and colored bright violet using aldehyde-fuchsin (Fig. 1A) or black using elastica-Masson (Fig. 1B). The nuchal skin dermis contained elastic fibers, which were identified as violet or black dots (Fig. 1C, D). We did not compare the direction of these fibers with that of other parts of the skin. The cartilages of the external auditory meatus, which were identified as irregularly shaped fragments, contained elastic fibers in the center of each fragment (Fig. 1E). The ligament-like structure between the cartilage fragments was also composed of elastic fibers (Fig. 1F, G). The morphology of the elastic fibers stained by these 2 methods did not differ critically. In addition, parotid gland acini and osteocytes in areas of endochondral ossification were darkly stained with aldehyde-fuchsin. Such clear discrimination of elastic fibers from other fibrous tissues by aldehyde-fuchsin staining was evident in 11 of the 15 specimens examined, while elastica-Masson staining consistently demonstrated elastic fibers in the arterial walls, skin dermis, and external ear cartilage. Therefore, most of the descriptions below are based on observations of these 11 specimens, for which both types of staining were possible.
The connective tissue around the maxilla and mandible, that is, the sphenomandibular ligament consistently showed elastic fibers (11/11), but they were restricted to the anterior layer of the superior half of the ligament (Fig. 2). Most of the fibers ran along the superoinferior axis (Fig. 2B, C). The temporomandibular joint disk also contained irregularly arrayed elastic fibers at the superolateral end (Fig. 2D). Although they were near or adjacent to the sphenomandibular ligament, we did not observe elastic fibers in the discomalleolar ligament. In the fascia covering striated muscles in the head, a fascia of the stylopharyngeus muscle specifically contained abundant elastic fibers (Fig. 2E-G); this fascia corresponded to the anterior margin of a large fatty tissue mass forming the prestyloid compartment of the parapharyngeal space or the prestyloid space [31, 32]. However, elastic fibers were found along the tensor veli palatini muscle or the primitive styloid process or Reichert's cartilage. The upper extension of the carotid sheath, as well as the prevertebral lamina of the deep cervical fascia, did not contain elastic fibers. In 3 of the 15 specimens, Meckel's cartilage contained elastic fibers in the anterior part of the perichondrium, but they were not stained with aldehydefuchsin (Fig. 2H-J). In addition, the periosteum of the mandible sometimes (3/11) contained elastic fibers at the lateral aspect facing the masseter muscle (Fig. 5C). The nasal mucosa contained a few elastic fibers along the periosteum of the hard palate (Fig. 5B).
We observed elastic fiber accumulation in ligament- or fascia-like structures that connected the hyoid bone, thyroid cartilage, and cricoid cartilage. Among these structures, the lateral thyrohyoid ligament (Fig. 3A-C) and the cricothyroid ligament (Fig. 3E) were almost purely composed of irregularly arrayed elastic fibers. The former connected the greater horn of the hyoid bone to the superior horn of the thyroid cartilage, while the latter connected the thyroid and cricoid cartilages along and near the anterior midline. These cartilages and the bones themselves were accompanied much or less elastic fibers in the perichondrium or periosteum. The epiglottic cartilage also contained elastic fibers, but they were interrupted by glands that invaded the cartilage. The vocal fold had not developed in the examined specimens. The submucosal tissue of the pharynx and larynx also contained a few elastic fibers. However, the laryngeal and pharyngeal wall muscles usually had no accompanying elastic fibers with 1 exception: abundant elastic fibers ran along the superior-inferior axis in a space between the palatopharyngeus muscle and the posterior pharyngeal mucosa (Fig. 3D). The submucosal tissue contained elastic fibers around the oral cavity, especially at the lateral angle near the opening of the parotid duct. The associated cartilage contained elastic fibers at and around the pharyngotympanic tube, but they were restricted to the junction between the lateral and medial laminae of the cartilage (Fig. 3F, G). The teeth were unaccompanied by elastic fibers.
Notably, elastic fibers had accumulated in the middle ear at 3 specific sites: 1) in the tendon of the stapedius muscle (Fig. 4A, B, E), 2) in the capsules of articulations between the malleus and the incus and between the incus and the stapes (Fig. 4C, D), and 3) in an interface area between the tensor tympani muscle and its tendon (Fig. 4F, G). Among these 3 sites, the latter 2 showed a constant morphology in all 11 examined specimens, which was in contrast with the observations of the joint capsules (3 of 11 specimens). However, elastic fibers in the joint capsule appeared to insert into the cartilage covering the ear ossicles. In the middle ear, the amount of elastic fibers was greatest in the tendon of the stapedius muscle. The epimysium of the middle ear muscles, the periosteum of the ear ossicles, and the tympanic membrane did not contain any elastic fibers. We failed to observe the base of the stapes attaching to the fenestra vestibuli. In addition, no elastic fibers were observed in the internal ear or eyeball.
In our observations, we did not find any clear differences between stages: individual differences in the strength of staining, especially with aldehyde-fuchsin appearing to mask any differences between stages. Because of our extensive trimming of the specimens, the face and eyelid could not be included. The orbitalis muscle behind the orbit was unaccompanied by elastic fibers (Fig. 5A). These results are summarized in Table 1.
This study revealed the distribution of elastic fibers in the fetal head and neck. It seems reasonable for elastic fibers to be abundant in ligament- or fascia-like structures in the larynx and pharynx because of functional demands during adulthood. At the same time, during development, these bands of elastic fibers appeared to maintain the topographical relationship between cartilages. Similar examples were seen in the external ear cartilages and the pharyngotympanic tube cartilage. In the former, cartilage fragments in the external meatus were united by elastic fiber bands as the meatus expanded. In the latter, the elastic fibers appeared to unite and fix the lateral lamina of the tubal cartilage to the medial lamina against traction by the tensor veli palatini muscle. The finding that the orbitalis muscle (the largest mass of smooth muscle in the fetus) contained no elastic fibers was unexpected; the muscle appeared to change into collagenous tissue through a process that differed from that of the sphenomandibular ligament (see below). Although there is little information about the distribution of elastic fibers in adults, we found that, in fetuses, 1) a mini-version of elastic fiber distribution compared to that in adults and 2) a different specific developmental pattern of connective tissues.
In fetal heads, Meckel's and Reichert's cartilages show a significant change in morphology as the final derivatives form [22-25]. Calcification can occur in either of these cartilages at specific sites such as the ear ossicles and styloid process. Changes in the histology of Meckel's cartilage have been well described in late-stage fetal and postnatal life [20, 21], but elastic fibers have received scant attention. Elastic fibers facilitate calcification because they accompany several proteins such as osteopontin that play a key role in bone and cartilage development as mentioned previously. Accordingly, these cartilages are likely to accompany elastic fibers in the intermediate developmental step before they attain their final morphology. Likewise, in the ligaments and fascia of the fetal head, the elastic fiber content is also likely to reflect an intermediate morphology between the cartilage and final collagen-dominant structure because elastic fibers are an important component of the scaffolds on which tissue development and remodeling depend [33, 34]. However, there has been no information about the elastic fiber contents of the adult sphenomandibular ligament and the posterior marginal fascia of the prestyloid space. The former ligament is a remnant of Meckel's cartilage [22], whereas we have hypothesized that the latter fascia is likely to be one of the derivatives of Reichert's cartilage (submitted as another paper). Shin et al. [35] termed the latter fascia "the tensor-vascular-styloid fascia." In contrast with the sphenomandibular ligament, we did not find elastic fibers in the adjacent discomalleolar ligament. However, if the elastic fibers represent an intermediate morphology during the differentiation of Meckel's cartilage, a further study seems necessary to identify elastin (not elastic fibers) in materials at a stage earlier than the present specimens.
The amount of elastin in tendons is usually limited to 0-2% of the dry mass, in contrast to 65-80% for collagens [36], with a few exceptions such as the vincular membrane in the phalanges of the hand [37] and tail tendons of animals [38]. The limited amount of elastin is thought to contribute to the recovery of wavy collagenous fibers after tendon stretching [36]. Likewise, ligaments contain few elastic fibers, with some exceptions such as the yellow ligament and nuchal ligament of the vertebral column [5, 39]. Notably, in this study, 2 striated muscles in the middle ear accompanied abundant elastic fibers at the muscle-tendon interface as well as in the tendon itself. Although striated muscle fibers have their own elasticity [40], the covering fascia of skeletal muscle contains various amounts of elastic fibers [41]. However, rather than elastic fibers, it is thought that the elasticity of the fascia is provided by overlaid collagenous meshworks, each displaying a different orientation. Thus, in contrast to smooth muscle (see Introduction), elastic fibers do not seem to play a critical role in the function of striated muscle, with a few exceptions such as the levator ani muscle at the pelvic floor [10, 42, 43]. We found that the middle ear muscles did not carry elastic fibers in either the epimysium or the covering fascia; this was not observed in the pelvic floor muscle. Under the specific conditions of constant vibration (the so-called acoustic oscillation) from the tympanic membrane, it seems reasonable that the elastic fibers in the tendons and joint capsules would reduce mechanical stress on the ear ossicles. The tendons attached to the ear ossicles develop through a complex process involving the regression of the pharyngeal arches [23, 25]. Thus, as hypothesized for elastic fibers in the aforementioned ligament and fascia, we cannot rule out the possibility that the elastic fiber content observed in the middle ear represents an intermediate morphology specific to late-stage fetuses.
Overall, the following were observed in human fetuses: 1) a mini-version of elastic fiber distribution compared to that in adults and 2) a different specific developmental pattern of connective tissues. Guadall et al. [44] reported that, during hypoxia, fibulin (a major component of microfilaments accompanying elastic fibers) is upregulated through a hypoxia-inducible factor 1-dependent mechanism. Thus, the latter morphology that was specific to fetuses might be the result of adaptation to hypoxic conditions during development. This study was limited in that the 2 types of staining that we employed were insufficient for identifying elastin or immature elastic fibers such as oxytalan and elaunin [45]. Because of the severe conditions used for decalcification, the present materials were unsuitable for elastin immunohistochemistry. When con sidering the intermediate morphology of the pharyngeal arch cartilages (see above), the immature status of the elastic fibers should be considered.
Figures and Tables
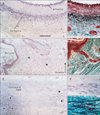 | Fig. 1Potential sites for elastic fibers in the fetal head. Sagittal sections. (A, C, E, F) Aldehyde-fuchsin staining. (B, D, G) Elastica-Masson staining. (A, B) The wall of the external carotid artery, (C, D) the skin covering the nuchal area, (E-G) the external auditory meatus (EAM). Panels (A) and (B) and panels (C) and (D) are pairs of consecutive sections. In (A) and (B), the internal elastic lamina (int lamina) is composed of circularly arranged elastic fibers, whereas the external lamina (ext lamina) is composed of sectioned longitudinal fibers identified as dots. In (C) and (D), the putative dermis (stars) contains abundant elastic fibers that run transversely. In (E), a cartilage fragment (cartilage of EAM) and a ligament between cartilages (stars) contain elastic fibers. Elastic fibers are accumulated near round cells in the center of the cartilage (arrows). Panels (F) and (G) show the ligament between cartilages in adjacent sections. Scale bar in (A)=0.1 mm (A-G). |
 | Fig. 2Elastic fibers in the ligaments and fasciae around the mandible. Sagittal sections. (A, C-E, G, H, J) Elastica-Masson staining. (B, F, I) Aldehyde-fuchsin staining. Panel (A) includes the sphenomandibular ligament (SML) and the lateral part of the disk (disk) of the temporomandibular joint. Panels (B) and (C, D) are higher-magnification views of the circles in (A). Elastic fibers are evident in the anterior layer of the SML (B, C) and the superolateral part of the joint disk (D). Panel (E) includes Reichert's cartilage (RC) and the stylopharyngeus and styloglossus muscles (SP and SG, respectively). Panels (F) and (G) are high-magnification views of the circle in (E). A fascia at the superior end of the SP contains abundant elastic fibers. Panel (H) includes Meckel's cartilage (MC) and the mandibular nerve root (MN). Panels (I) and ( J) are high-magnification views of the circle in (H). The anterior part of the perichondrium of MC contains elastic fibers ( J), but they are not stained with aldehydefuchsin (I). ECA, external carotid artery; LP, lateral pterygoid muscle; MA, masseter muscle; PS, prestyloid compartment of the parapharyngeal space. The periosteum of the mandible, see Fig. 5C. Scale bars=1 mm (A, E, H), 0.1 mm (B-D, F, G, I, J). |
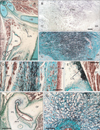 | Fig. 3Elastic fibers in the larynx, pharynx, and surrounding areas. Sagittal sections. (A, C-G) Elastica-Masson staining. (B) Aldehyde-fuchsin staining. Panel (A) includes the body and greater horn of the hyoid bone (HB and GH, respectively) and the thyroid cartilage (TC). Panels (B) and (C) are high-magnification views of the circle in (A). The lateral thyrohyoid ligament (LTHL), connecting the greater horn (GH) to the superior horn (SH) of the TC, is composed of randomly arrayed elastic fibers. Panel (D) corresponds to the circle marked D in (A). Elastic fibers run along the superior-inferior axis in a space between the palatopharyngeus muscle (PP) and the posterior pharyngeal mucosa. Panel (E) displays the cricothyroid ligament (CTL) immediately above the cricoid cartilage (CC). Panel (F) shows the pharyngotympanic tube (PTT) and its associated cartilage. Panel (G) is a high-magnification view of the circle in (F). Elastic fibers are evident at the junction between the lateral and medial laminae (LL and ML, respectively) of the cartilage. CAP, cricoarytenoideus posterior muscle; CPI, constrictor pharyngis inferior muscle; OF, Ostmann's fat; PC, pharyngeal cavity; SNL, superior laryngeal nerve;ST, sternothyroideus muscle; TVP, tensor veli palatini muscle. Scale bars=1 mm (A, F), 0.1 mm (B-E, G). |
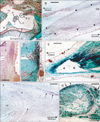 | Fig. 4Elastic fibers along and around the ear ossicles. Sagittal sections. (A, D, E, G) Elastica-Masson staining. (B, C, F) Aldehyde-fuchsin staining. Panel (A) displays the tympanic cavity including the malleus (M), incus (I), stapes (S), and tympanic membrane (tympanic m). Panels (B) and (E) are high-magnification views of the circle marked B, E in (A) and display the muscle-tendon interface of the stapedius muscle (SM). Asterisks in (E) indicate damage sustained during the histological procedure. Panels (C) and (D) show part of the incudostapedial joint that corresponds to the circle marked C, D in (A). Panels (F) and (G) display the interface area between the tensor tympani muscle (TT) and its tendon. Elastic fibers are located around the tendon (arrows) and at the interface (stars). FN, facial nerve; RC, Reichert's cartilage. Scale bars=1 mm (A), 0.1 mm (B-G). |
 | Fig. 5Other areas of the head. Sagittal sections. (A-C) Elastica-Masson staining. Panel (A) shows the orbitalis muscle, which contains no elastic fibers. Panel (B) shows elastic fibers in the submucosal layer (double-headed arrow) of the nose near the bony palate periosteum. Panel (C) shows elastic fibers in the periosteum of the mandible facing the masseter muscle (MA). Scale bar in (A)=0.1 mm (A-C). |
Acknowledgements
We are grateful to Mr. Hiroyuki Oosugi (Department of Anatomy, Okayama University School of Medicine) for his assistance with elastic fiber staining.
References
1. Dingemans KP, Teeling P, Lagendijk JH, Becker AE. Extracellular matrix of the human aortic media: an ultrastructural histochemical and immunohistochemical study of the adult aortic media. Anat Rec. 2000. 258:1–14.
2. Kono R, Poukens V, Demer JL. Quantitative analysis of the structure of the human extraocular muscle pulley system. Invest Ophthalmol Vis Sci. 2002. 43:2923–2932.
3. Osanai H, Murakami G, Ohtsuka A, Suzuki D, Nakagawa T, Tatsumi H. Histotopographical study of human periocular elastic fibers using aldehyde-fuchsin staining with special reference to the sleeve and pulley system for extraocular rectus muscles. Anat Sci Int. 2009. 84:129–140.
4. Osanai H, Abe S, Rodríguez-Vázquez J, Verdugo-López S, Murakami G, Ohguro H. Human orbital muscle: a new point of view from the fetal development of extraocular connective tissues. Invest Ophthalmol Vis Sci. 2011. 52:1501–1506.
5. Fukuda Y, Ferrans VJ, Crystal RG. Development of elastic fibers of nuchal ligament, aorta, and lung of fetal and post natal sheep: an ultrastructural and electron microscopic immunohistochemical study. Am J Anat. 1984. 170:597–629.
6. Nakamura T, Hashimoto N, Maeda Y, Ikeda T, Nakagawa H, Takagi K. Degeneration and ossification of the yellow ligament in unstable spine. J Spinal Disord. 1990. 3:288–292.
7. Viejo-Fuertes D, Liguoro D, Rivel J, Midy D, Guerin J. Morphologic and histologic study of the ligamentum flavum in the thoraco-lumbar region. Surg Radiol Anat. 1998. 20:171–176.
8. Pasquali-Ronchetti I, Baccarani-Contri M. Elastic fiber during development and aging. Microsc Res Tech. 1997. 38:428–435.
9. Shiraishi Y, Kobayashi M, Yasui M, Ozaki N, Sugiura Y. Innervation and functional characteristics of connective tissues, especially elastic fibers, in human fetal thoracic intervertebral articular capsule and its surroundings. Anat Embryol (Berl). 2003. 206:437–445.
10. Hirata E, Fujiwara H, Hayashi S, Ohtsuka A, Abe S, Murakami G, Kudo Y. Intergender differences in histological architecture of the fascia pelvis parietalis: a cadaveric study. Clin Anat. 2011. 24:469–477.
11. Kato M, Matsubara A, Murakami G, Abe S, Ide Y, Sato I, Usui T. Female perineal membrane: a study using pelvic floor semiserial sections from elderly nulliparous and multiparous women. Int Urogynecol J Pelvic Floor Dysfunct. 2008. 19:1663–1670.
12. Kinugasa Y, Arakawa T, Abe S, Ohtsuka A, Suzuki D, Murakami G, Fujimiya M, Sugihara K. Anatomical reevaluation of the anococcygeal ligament and its surgical relevance. Dis Colon Rectum. 2011. 54:232–237.
13. Nagata I, Murakami G, Suzuki D, Furuya K, Koyama M, Ohtsuka A. Histological features of the rectovaginal septum in elderly women and a proposal for posterior vaginal defect repair. Int Urogynecol J Pelvic Floor Dysfunct. 2007. 18:863–868.
14. Nagy NB, Daniel JC. Distribution of elastic fibres in the developing rabbit craniomandibular joint. Arch Oral Biol. 1991. 36:15–23.
15. O'Dell NL, Starcher BC, Wilson JT, Pennington CB, Jones GA. Morphological and biochemical evidence for elastic fibres in the Syrian hamster temporomandibular joint disc. Arch Oral Biol. 1990. 35:807–811.
16. Ueki S, Iwai-Liao Y, Tsubai T, Kojima H, Higashi Y. Fine structure of cartilage elastic system fibers, in particular those of the mandibular condyle. J Osaka Dent Univ. 1989. 23:99–109.
17. Caltabiano M, Caltabiano C, Martinez G, Leonardi R. Histology of the cartilage of the human fetal condyle. Mondo Ortod. 1990. 15:439–442.
18. Baccarani-Contri M, Taparelli F, Pasquali-Ronchetti I. Osteopontin is a constitutive component of normal elastic fibers in human skin and aorta. Matrix Biol. 1995. 14:553–560.
19. Perrotta I, Russo E, Camastra C, Filice G, Di Mizio G, Colosimo F, Ricci P, Tripepi S, Amorosi A, Triumbari F, Donato G. New evidence for a critical role of elastin in calcification of native heart valves: immunohistochemical and ultrastructural study with literature review. Histopathology. 2011. 59:504–513.
20. Harada Y, Ishizeki K. Evidence for transformation of chondrocytes and site-specific resorption during the degradation of Meckel's cartilage. Anat Embryol (Berl). 1998. 197:439–450.
21. Richany SF, Bast TH, Anson BJ. The development of the first branchial arch in man and the fate of Meckel's cartilage. Q Bull Northwest Univ Med Sch. 1956. 30:331–355.
22. Rodríguez Vázquez JF, Merida Velasco JR, Jimenez Collado J. Development of the human sphenomandibular ligament. Anat Rec. 1992. 233:453–460.
23. Rodríguez-Vázquez JF. Development of the stapes and associated structures in human embryos. J Anat. 2005. 207:165–173.
24. Rodriguez-Vazquez JF, Mérida-Velasco JR, Verdugo-López S, Sánchez-Montesinos I, Mérida-Velasco JA. Morphogenesis of the second pharyngeal arch cartilage (Reichert's cartilage) in human embryos. J Anat. 2006. 208:179–189.
25. Rodríguez-Vázquez JF. Development of the stapedius muscle and pyramidal eminence in humans. J Anat. 2009. 215:292–299.
26. Fujita T. Histological studies on the neuro-insular complex in the pancreas of some mammals. Z Zellforsch. 1959. 50:94–109.
27. Sunami-Kataoka Y, Akagi H, Nishizaki K, Taguchi T, Murakami T, Ohtsuka A. Chondroitin sulfate proteoglycan at the basal lamina beneath high endothelial cells in human palatine tonsils: a light and electron microscopic study using the cationic colloidal iron method. Arch Histol Cytol. 2001. 64:535–543.
28. Hayashi T, Kumasaka T, Mitani K, Yao T, Suda K, Seyama K. Loss of heterozygosity on tuberous sclerosis complex genes in multifocal micronodular pneumocyte hyperplasia. Mod Pathol. 2010. 23:1251–1260.
29. Motohashi O, Suzuki M, Shida N, Umezawa K, Ohtoh T, Sakurai Y, Yoshimoto T. Subarachnoid haemorrhage induced proliferation of leptomeningeal cells and deposition of extracellular matrices in the arachnoid granulations and subarachnoid space. Immunhistochemical study. Acta Neurochir (Wien). 1995. 136:88–91.
30. Okeda R, Arima K, Kawai M. Arterial changes in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) in relation to pathogenesis of diffuse myelin loss of cerebral white matter: examination of cerebral medullary arteries by reconstruction of serial sections of an autopsy case. Stroke. 2002. 33:2565–2569.
31. Chong VF, Mukherji SK, Goh CH. The suprahyoid neck: normal and pathological anatomy. J Laryngol Otol. 1999. 113:501–508.
32. Li QY, Zhang SX, Liu ZJ, Tan LW, Qiu MG, Li K, Cui GY, Guo YL, Yang XP, Zhang WG, Chen XH, Chen JH, Ding SY, Chen W, You J, Wang YS, Deng JH, Tang ZS. The pre-styloid compartment of the parapharyngeal space: a three-dimensional digitized model based on the Chinese Visible Human. Surg Radiol Anat. 2004. 26:411–416.
33. Christov A, Korol RM, Dai E, Liu L, Guan H, Bernards MA, Cavers PB, Susko D, Lucas A. In vivo optical analysis of quantitative changes in collagen and elastin during arterial remodeling. Photochem Photobiol. 2005. 81:457–466.
34. Zheng Q, Choi J, Rouleau L, Leask RL, Richardson JA, Davis EC, Yanagisawa H. Normal wound healing in mice deficient for fibulin-5, an elastin binding protein essential for dermal elastic fiber assembly. J Invest Dermatol. 2006. 126:2707–2714.
35. Shin JH, Lee HK, Kim SY, Choi CG, Suh DC. Imaging of parapharyngeal space lesions: focus on the prestyloid compartment. AJR Am J Roentgenol. 2001. 177:1465–1470.
36. Kannus P. Structure of the tendon connective tissue. Scand J Med Sci Sports. 2000. 10:312–320.
37. Ritty TM, Ditsios K, Starcher BC. Distribution of the elastic fiber and associated proteins in flexor tendon reflects function. Anat Rec. 2002. 268:430–440.
38. Korol RM, Finlay HM, Josseau MJ, Lucas AR, Canham PB. Fluorescence spectroscopy and birefringence of molecular changes in maturing rat tail tendon. J Biomed Opt. 2007. 12:024011.
39. Gleason PD, Beall DP, Sanders TG, Bond JL, Ly JQ, Holland LL, Pasque CB. The transverse humeral ligament: a separate anatomical structure or a continuation of the osseous attachment of the rotator cuff? Am J Sports Med. 2006. 34:72–77.
40. Ochala J, Frontera WR, Dorer DJ, Van Hoecke J, Krivickas LS. Single skeletal muscle fiber elastic and contractile characteristics in young and older men. J Gerontol A Biol Sci Med Sci. 2007. 62:375–381.
41. Stecco C, Porzionato A, Macchi V, Tiengo C, Parenti A, Aldegheri R, Delmas V, De Caro R. Histological characteristics of the deep fascia of the upper limb. Ital J Anat Embryol. 2006. 111:105–110.
42. Delancey JO. Fascial and muscular abnormalities in women with urethral hypermobility and anterior vaginal wall prolapse. Am J Obstet Gynecol. 2002. 187:93–98.
43. Hirata E, Koyama M, Murakami G, Ohtsuka A, Abe S, Ide Y, Fujiwara H, Kudo Y. Comparative histological study of levels 1-3 supportive tissues using pelvic floor semiserial sections from elderly nulliparous and multiparous women. J Obstet Gynaecol Res. 2011. 37:13–23.
44. Guadall A, Orriols M, Rodríguez-Calvo R, Calvayrac O, Crespo J, Aledo R, Martínez-Gonzalez J, Rodríguez C. Fibulin-5 is upregulated by hypoxia in endothelial cells through a hypoxia-inducible factor-1 (HIF-1alpha)-dependent mechanism. J Biol Chem. 2011. 286:7093–7103.
45. Cotta-Pereira G, Guerra Rodrigo F, Bittencourt-Sampaio S. Oxytalan, elaunin, and elastic fibers in the human skin. J Invest Dermatol. 1976. 66:143–148.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download