Abstract
Lead, one of the most toxic heavy metals, takes longer time to be excreted from the body than other heavy metals. The purpose of this study is, by measuring lead excretion via urine and feces, to find out the effect of vitamin C in lead chelation. Thirty-six rats were randomly assorted into four groups. All 33 rats except for the control group were administered with lead, before orally administered with different doses of vitamin C per kilogram of body weight. The lead excretion levels in urine and feces as well as the survival rate were then measured for each group. The rats with lead administrations (10/13, 76.9%) with lead administrations only, 10/11 rats (90.9%) with lead administrations and low dose of vitamin C, 9/9 rats (100%) with lead administrations and high dose of vitamin C survived. Among the 29 surviving rats, low vitamin C intake group exhibited higher urinary excretion than the lead only group. The urinary excretion level in high dose vitamin C intakegroup was significantly higher than the lead only group. In addition, fecal lead excretion seemed to be increased in the high dose vitamin C intake group, compared to the group with lead administrations only with statistical significance. Through animal experiment, it was found out that administrating high dose of vitamin C accelerated the excretion of lead in body compared to low dose of vitamin C.
Lead is currently used in a variety of applications including storage battery, water pipe, soldering, paint, and plastic. Due to commercial applications, the use of lead increased markedly in the 1940s, exceeding the total amount of lead used in all previous centuries. After the expansion of its use in the 1940s, lead was emitted in particle form, causing extensiveair, dust, soil, drinking water, and crop contamination and ultimately resulting inincreased blood concentrations of lead in the general public.
Lead is known to have some toxic effects on membrane structure and functions of various cells [1]. Particularly, the effects on membranes are intensely analyzed, because red blood cells have a high affinity for lead. A majority of the lead-contaminated cells in the bloodstream has been more vulnerable to oxidative damage than many other cells [2, 3]. Osmotic and mechanic susceptibilities of red blood cells were reported to have increased lead toxicity [4], accompanied by decreased deformability and a shortened life span [5-7].
Chelating agents that are used to cure lead poisoning include mesodimercaptosuccinic acid (DMSA), dimercaptopropan-1-sulphonate, and ethylendiamine tetraacetic acid [8, 9]. Chelating agents couple with heavy metals, such as lead and mercury, and enhance their excretion in sweat and urine. It is not known if chelating agents are harmful to the human body.
There have been studies reporting that a number of antioxidant substances including harmless vitamin C prevent the accumulation of heavy metals. It is estimated in the study of rabbit's renal cortex that vitamin C prevents the accumulation of paraquat by the ascorbic acid mediated transmembrane-reducing system [10]. Also, it has been reported that vitamin C does not increase the impact of DMSA in the rat exposed to lead [9], but there have been few data about human subjects. Simon et al examined the relationship between vitamin C and lead in blood by conducting cross-sectional study against 4,213 children from 1988 to 1994. They reported that the high level of vitamin C in blood is independently related with the decrease of the prevalence rate of the high concentration of lead in blood [11]. Besides, the role of vitamin C as an antioxidant has already been recognized [12]. Antioxidant nutrients such as vitamin E, vitamin B6, β-carotene, zinc, and selenium other than vitamin C are also effective for oxidative stress caused by lead poisoning [12].
There are studies reporting that the administration of vitamin C with thiamine accelerates the excretion of lead through urine significantly, reduces the accumulation of lead in liver and kidney, and reduces the interference of lead on the delta-aminolevulinic acid dehydratase action of blood [13]. It has been reported also that the use of vitamin C with thiol chelators such as DMSA reduces oxidative stress significantly [14-16], although it is no more effective than the treatment with thiol chelator only [9, 14].
The aim of this study was to determine the effect of vitamin C on lead excretion. The inhibition of gastrointestinal lead absorption was determined by administering vitamin C to lead-exposed rats and then measuring the fecal lead excretion. The effect of vitamin C on the discharge of lead that was deposited in the blood and organs was investigated via measurement of the urinary excretion of lead. Although there are studies reporting the protective role of vitamin C to lead poisoning, there are no reports about effective amount of vitamin C. Also, since there were no reports regarding the proper dose of vitamin C for the facilitation of lead excretion, we created a low-dose vitamin C group and a high-dose vitamin C group in an attempt to determine if increased vitamin C levels correlate with increase lead excretion.
Thirty-six Sprague-Dawley male rats aged 8 weeks and weighing 250 g in average were purchased from Samtako Bio Korea (Osan, Korea) and adapted to the animal laboratory environment by placing 4 rats in each Plexiglas cage without windows for 3 days while keeping the temperature at 22±1℃ and turning on the light at 7 AM and off at 9 PM in the Animal Laboratory of the College of Medicine, Kosin University before the test. They were fed with standard laboratory chow and water ad lib. Metabolic cages facilitated the separate collection of urine and feces. All research procedures involving animals were performed in accordance with the guidelines for the Animal Welfare Committee at College of Medicine, Kosin University.
For vitamin C, we used a solution (L-ascorbic acid) manufactured by Unimed Co. Ltd. (Asan, Korea) was used. For the lead treatment, we used the Lead Atomic Absorption Standard Solution (100 µg Pb/ml in 1% HNO3, 100 ml) available from Sigma (St. Louis, MO, USA).
A total 36 male rats were tested for eight days. They were divided into 4 groups: a control group with no lead administration (n=3); a group with 0.2 mg/kg/day of lead chloride solution only without any vitamin C (n=13); a group with administered with a low dose of vitamin C solution (0.5 g/kg/day) after the lead administration (n=11); and a group administered with a high dose of vitamin C solution (2 g/kg/day) after the lead administration (n=9). The amount of lead administered was equal in every group. Appropriate amount of lead and vitamin C for the rats was referred to the papers about the effect of vitamin C on animal testing [9, 14, 17]. To measure the excretion of lead through urine and feces, urine and feces were collected on alternate days (the day before, 2nd day, 4th day, 6th day, and 8th day) from 3 rats in the control group, 10 rats in the lead only group, 10 rats in the low dose vitamin C group, and 9 rats in the high dose vitamin C group. Rats that died during the test period were removed from the analysis, which include 3 in the lead only group and 1 in the low dose vitamin C group (Figs. 1, 2).
Lead and vitamin C solutions were orally administered everyday at 2 PM, into the stomach with a 10 cm Oral Zonde Needle (NATUME, Sendai, Japan) under the anesthesia and vitamin C was administered from the day before for 8 days and lead was administered before vitamin C. Administration five hundred milligram of vitamin C of per rat's weight (kg) mixed with 1 ml of physiological saline was administered to the low dose vitamin C group and 2 g of vitamin C of per rat's weight (kg) mixed with 1 ml of physiological saline was administered to the high-dose vitamin C group. The lead of 0.4 mg of per rat's weight (kg) mixed with 1 ml of physiological saline was administered to each group.
To reduce incidences of rats dying from the oral needle administrations, a preliminary test, in which physiological saline was administered orally to 10 separate rats using Oral Zonde Needle, was conducted for 2 weeks before the test. One of the members participated in the preliminary test continued the rest of the study. The result did not show any finding that implies aspiration during oral administration or injury such as bleeding. No autopsy was conducted separately.
Urine and feces were collected once every two days at 10 AM. For the test, feces were retrieved, placed in 100 ml of physiological saline, crushed by using Mini Shaker (Winbiotech, Bucheon, Korea), filtered with filter paper for 5 minutes, placed in 15 ml test tube, and stored for 1 day. The urine was placed in the tube, stored for 1 day, and sent to the analytical laboratory at 4 PM. The body weight of each rat was also measured on the same date. The analysis was made with Analyst 800, an atomic absorption spectrometer by Perkin Elmer (Santa Clara, CA, USA), and the wavelength was 283.3 nm, lamp current was 5 mA, slit width was 0.5 nm, and slit height was normal, and Zeeman background correction was made. The specimen of 20 µl of specimen was injected into the graphite tube using automatic specimen injector. The mode of method was summarized in the Table 1.
The average weight of each group increased gradually. The P-value from the comparison of the weight change with repeated measure ANOVA was 0.341, suggesting that there was no significant difference among groups (Table 2).
In the control group, which included three rats that did not receive any lead administration, all subjects survived. Ten rats (76.9%) that were administered lead only, 10/11 rats (90.9%) that were administered lead and low-dose of vitamin C, 9/9 rats (100%) that were administered lead and high-dose of vitamin C survived and were used for analysis.
The average lead excretion amounts in urine in each group by dates were shown in Table 3. As shown in this table. The lead excretion amount was increased in vitamin C groups compared with the lead only group, and in particular, it was further increased in high dose vitamin C group. Also, it was found that the total excretion of lead in urine was higher in high dose vitamin C group than those of other groups. We could see that urinary lead excretion among the 29 surviving rats. Results apart from 3 in control group indicated a significance difference in each group in the analysis of ANOVA test (P<0.001). With post-hoc test, urinary lead excretion in low dose vitamin C group was higher than the excretion in lead only group, but it was statistically insignificant. The urinary excretion level in high dose vitamin C group was significantly higher than the lead only group's (P<0.001) (Table 4, Fig. 2). Also, compared to low dose vitamin C group, lead excretion in high dose vitamin C group was significantly elevated (P=0.003) (Table 4, Fig. 3).
The average fecal lead excretion amounts by date are shown in Table 5. Lead excretion in feces among the 29 surviving rats, apart from 3 in control group were different in each group through repeated measure ANOVA (P=0.046). With post-hoc test, Lead excretion in feces in low dose vitamin C group was higher than the excretion in lead only group, but it was statistically insignificant (P=0.282). The fecal excretion level in high dose vitamin C group was significantly high (P=0.049) (Table 6, Fig. 4). Also, compared to low dose vitamin C group, lead excretion in high dose vitamin C group was increased but not statistically meaningful (Table 6, Fig. 4).
The total amount of lead administration was equal in each group (875 µg). The combined urinary and fecal lead excretion was 230.7 µg (26.37%) in the lead only group, 568.08 µg (64.92%) in the lead and low-dose vitamin C group, and 860.03 µg (98.29%) in the lead and high-dose vitamin C group.
The control group, three rats without any lead administration, all survived and 10/13 rats (76.9%) with lead administrations only, 10/11 rats (90.9%) with lead administrations and low-dose of vitamin C, 9/9 rats (100%) with lead administrations and high-dose of vitamin C survived. Among the 29 surviving rats, except three in control group, urinary lead excretion in low-dose vitamin C group (82.43 µg) was higher than the excretion in lead only group (60.87 µg), but it was statistically insignificant. The urinary excretion level in high-dose vitamin C group (276.13 µg) was significantly high (P<0.001) compared to the control group. Similarly fecal excretion level in high-dose vitamin C group (583.90 µg) was significantly higher than the excretion in lead only group (169.83 µg) (P=0.049).
This study has several limitations. First, there was not an equal number of rats in each group (low-vitamin C, high-dose vitamin C, lead only, and control). A higher percentage of rats that were administered with lead only were in danger of dying during the experiment. Therefore, 13 rats, compared to 9 to 10 rats administered with both lead and vitamin C, were used to perform the test. Second, the actual change in the concentration of lead in the body was not determined because measurement of lead level in blood or autopsy of the organs in which the lead is likely to be accumulated was not performed. Third, although to our knowledge the current study measured fecal lead levels for the first time, the validity of the method for retrieving feces has not been confirmed. Finally, we were unable to detect minute changes in lead excretion because the fecal and urinary lead concentration were not measured on a daily basis.
The administration of vitamin C with thiamine accelerates the excretion of lead through urine significantly, reduces the accumulation of lead in liver and kidney, and reduces the interference of lead on the delta-aminolevulinic acid dehydratase action of blood [13] and it has been reported also that the use of vitamin C with thiol chelators such as DMSA reduces oxidative stress significantly [14-16] although it is no more effective than the treatment with thiol chelator only [9, 14]. Antioxidant nutrients such as vitamin E, vitamin B6, β-carotene, zinc, and selenium other than vitamin C are also effective for oxidative stress caused by lead poisoning [12] and therefore co-administration of these nutrients with vitamin C shall be considered also.
To find out the use of vitamin C, studies so far have examined lead excretion via urine or lead accumulation in organs. So they didn't measure total amount of excretion (urine and stool), even though measured some lead excretion by vitamin C. However, for the first time in this test, to know the amount of excretion through bile and absorption through intestine, total lead excretion via urine and stool was measured. Since, there has been no study to see effective dosage of vitamin C for lead excretion, through this experiment, we could see that high dose of vitamin C was more effective than low dose in lead excretion. Additional studies seem necessary to find the most effective dosage to human, since animal experiment cannot be same as human's.
Part of vitamin C's effective clinical neutralization of lead may well involve lead's storage in a much less toxic form due to its interaction with vitamin C. Indeed, this is one very good reason why a great deal of caution must be exerted when embarking upon a brisk detoxification program. Lead and a host of other stored toxins can be readily mobilized from storage site with a number of different detoxification agents. It seems that urinary and fecal lead excretion through lymph, bloodstream and bile increase when lead transform from immobilized to mobilized form by vitamin C.
The average lead excretion amounts in urine in each group by dates were shown in Table 3. In high dose vitamin C group, urinary lead excretion started to increase at the beginning and drastically increased through 4th and 6th day. At 8th day, excretion was still high. However in low dose vitamin C group, it did increase lead excretion, but we could clearly see the difference with high dose vitamin C group.
The average lead excretion amounts in feces by dates were shown in Table 5. The decrease of excretion on the 6th and 8th day appears to have relevance to the fact that a large amount of lead accumulated inside the body has already been excreted and only small amount of it remained inside the body, and in Fig. 3, vitamin C groups excreted more lead than the amount of lead that was administered on the same date. On the 4th day, the lead only group excreted more lead than the amount administered on the same date, however, less than the daily administration afterwards. In other words, the decrease in the excretion of lead in the lead only group from the 6th day appears to be influenced by the limit of rat's own capacity to excrete lead unlike that in vitamin C groups, and it suggests that the accumulation of lead will grow seriously eventually.
Since the result of paired t-test showed the significant excretion of lead in urine and feces from the day after the administration of lead, particularly in high dose vitamin C group, the administration of vitamin C for preventing lead poisoning can be considered.
Therefore, vitamin C could be used as an important alternative medicine for the treatment of lead poisoning at least, and more importantly, the use of high dose vitamin C as a safe and independent cure for heavy metal poisoning as it is harmless to human body shall be taken into consideration compared with existing chelators whose adverse effect on human body is not clearly known. Also in this study, when using high dose vitamin C, the injection will be naturally more efficient than oral administration. Administering vitamin C, particularly high-dose vitamin C increased the excretion of lead in urine and it is expected to prevent the accumulation of lead inside the body effectively.
The future investigation of the preventive impact of vitamin C shall involve tests such as the measurement of the excretion of lead in rats to which vitamin C was administered for a long time before administering lead and studies such as the measurement of the concentration of lead in the tissue and measurement of concentration of vitamin C in the peripheral blood after oral administration.
Through this experimental study, we found that administrating high-dose of vitamin C accelerated excretion of lead by inhibiting lead absorption in intestine and increasing excretion form blood and organs and then, most of the lead administered in high-dose vitamin C group (98.29%) was excreted via urine and feces. Amount of lead excretion was proportional to vitamin C intake. Since the effective vitamin C dose for the facilitation of lead excretion in rats is not likely to be the same as that for humans, additional studies are needed to determine the most appropriate dose for humans.
Figures and Tables
Fig. 1
Experimental schedule of vitamin C (Vit. C) and lead administration and of retrieving lead from urine and feces. To measure the excretion of lead through urine and feces, urine and feces were collected on alternate days (the day before, 2nd day, 4th day, 6th day, and 8th day). To see the effect of Vit. C on suppressing intestinal absorption of lead, Vit. C was started to administrate a day before lead administration.
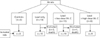
Fig. 2
Experimental flow. Overall 32 rats were included in experiment; 3 rats died in a group of lead only; 1 rat died in a group of lead and low dose vitamin C.

Fig. 3
Lead excretion in urine. In high dose vitamin C group, urinary lead excretion started to increase at the beginning and drastically increased through 4th and 6th day. At 8th day, excretion was still high. However in low dose vitamin C group, it did increase lead excretion, but we could clearly see the difference with high dose vitamin C group.

Fig. 4
Lead excretion in stool. The decrease of excretion on the 6th and 8th day appears to have relevance to the fact that a large amount of lead accumulated inside the body has already been excreted and only small amount of it remained inside the body, vitamin C groups excreted more lead than the amount of lead that was administered on the same date. On the 4th day, the lead only group excreted more lead than the amount administered on the same date, however, less than the daily administration afterwards. In other words, the decrease in the excretion of lead in the lead only group from the 6th day appears to be influenced by the limit of rat's own capacity to excrete lead unlike that in vitamin C groups, and it suggests that the accumulation of lead will grow seriously eventually.

References
1. Donaldson WE, Knowles SO. Is lead toxicosis a reflection of altered fatty acid composition of membranes? Comp Biochem Physiol C. 1993; 104:377–379.
2. deSilva PE. Determination of lead in plasma and studies on its relationship to lead in erythrocytes. Br J Ind Med. 1981; 38:209–217.
3. Leggett RW. An age-specific kinetic model of lead metabolism in humans. Environ Health Perspect. 1993; 101:598–616.
4. Waldron HA. The anaemia of lead poisoning: a review. Br J Ind Med. 1966; 23:83–100.
5. Hernberg S, Nurminen M, Hasan J. Nonrandom shortening of red cell survival times in men exposed to lead. Environ Res. 1967; 1:247–261.
6. Levander OA, Morris VC, Ferretti RJ. Filterability of erythrocytes from vitamin E-deficient lead-poisoned rats. J Nutr. 1977; 107:363–372.
7. Lidsky TI, Schneider JS. Adverse effects of childhood lead poisoning: the clinical neuropsychological perspective. Environ Res. 2006; 100:284–293.
8. Piomelli S, Rosen JF, Chisolm JJ Jr, Graef JW. Management of childhood lead poisoning. J Pediatr. 1984; 105:523–532.
9. Varnai VM, Piasek M, Blanusa M, Juresa D, Sarić M, Kostial K. Ascorbic acid supplementation does not improve efficacy of meso-dimercaptosuccinic acid treatment in lead-exposed suckling rats. Pharmacol Toxicol. 2003; 93:180–185.
10. Fujimoto Y, Nakatani E, Horinouchi M, Okamoto K, Sakuma S, Fujita T. Inhibition of paraquat accumulation in rabbit kidney cortex slices by ascorbic acid. Res Commun Chem Pathol Pharmacol. 1989; 65:245–248.
11. Simon JA, Hudes ES. Relationship of ascorbic acid to blood lead levels. JAMA. 1999; 281:2289–2293.
12. Hsu PC, Guo YL. Antioxidant nutrients and lead toxicity. Toxicology. 2002; 180:33–44.
13. Gurer H, Ercal N. Can antioxidants be beneficial in the treatment of lead poisoning? Free Radic Biol Med. 2000; 29:927–945.
14. Flora SJ, Pande M, Mehta A. Beneficial effect of combined administration of some naturally occurring antioxidants (vitamins) and thiol chelators in the treatment of chronic lead intoxication. Chem Biol Interact. 2003; 145:267–280.
15. Patra RC, Swarup D, Dwivedi SK. Antioxidant effects of alpha tocopherol, ascorbic acid and L-methionine on lead induced oxidative stress to the liver, kidney and brain in rats. Toxicology. 2001; 162:81–88.
16. Tandon SK, Singh S, Prasad S, Srivastava S, Siddiqui MK. Reversal of lead-induced oxidative stress by chelating agent, antioxidant, or their combination in the rat. Environ Res. 2002; 90:61–66.
17. Acharya UR, Rathore RM, Mishra M. Role of vitamin C on lead acetate induced spermatogenesis in swiss mice. Environ Toxicol Pharmacol. 2003; 13:9–14.




 PDF
PDF ePub
ePub Citation
Citation Print
Print








 XML Download
XML Download