Abstract
Hypoxia-ischemia leads to serious neuronal damage in some brain regions and is a strong risk factor for stroke. The aim of this study was to investigate the neuroprotective effect of tanshinone I (TsI) derived from Danshen (Radix Salvia miltiorrhiza root extract) against neuronal damage using a mouse model of cerebral hypoxia-ischemia. Brain infarction and neuronal damage were examined using 2,3,5-triphenyltetrazolium chloride (TTC) staining, hematoxylin and eosin histochemistry, and Fluoro-Jade B histofluorescence. Pre-treatment with TsI (10 mg/kg) was associated with a significant reduction in infarct volume 1 day after hypoxia-ischemia was induced. In addition, TsI protected against hypoxia-ischemia-induced neuronal death in the ipsilateral region. Our present findings suggest that TsI has strong potential for neuroprotection against hypoxic-ischemic damage. These results may be used in research into new anti-stroke medications.
For several decades, the application of complementary and alternative medicines using Chinese herbs in ischemic stroke has been a subject of intense interest, and many studies have been conducted to investigate the effect of plant extracts on the brains of animal models with ischemic insults [1-3]. Some of these herbal medicines have been used for centuries without demonstrating significant adverse effects in humans; therefore, their active ingredients may serve as efficacious and safe candidates for prevention of ischemic stroke [4, 5].
Danshen (Radix Salvia miltiorrhiza root) extract is widely used in oriental medicine for the treatment of various microcirculatory disturbance-related conditions, including cardiovascular and cerebrovascular diseases [6, 7]. The main lipophilic diterpenoid quinines in Danshen are tanshinones, including cryptotanshinone, dihydrotanshinone I, tanshinone I (TsI), tanshinone IIA (TsIIA), and tanshinone IIB (TsIIB) [8]. Tanshinones have the potential to penetrate the blood-brain barrier [9], and have been reported to exert antioxidant and anti-inflammatory effects in the prevention of ischemic injury in animal models [10, 11].
Although some researchers have focused on the protective effects of TsIIA and/or TsIIB in transient focal/global cerebral ischemia [12-14], the potential neuroprotective effect of TsI after occurrence of hypoxia-ischemia has not been studied. Therefore, in the present study, we aimed to assess the neuroprotective effect of TsI in the brain of a mouse model with hypoxia-ischemia, which is different from transient cerebral ischemia in terms of the process of neuronal damage.
The roots of S. miltiorrhiza Bunge (Labiatae) were purchased in March 2005 at the University Oriental Herbal Drugstore, Iksan, Korea, and their identity was verified by Dr. Kyu-Kwan Jang of the Botanical Garden, Wonkwang University. A voucher specimen (no. WP05-87) was deposited at the herbarium of the College of Pharmacy, Wonkwang University (Korea). Dried and pulverized roots of S. miltiorrhiza (2 kg) were soaked in 1.6 l distilled water for 12 hours at room temperature, extracted with hot ethanol for 2 hours, and filtered with filter paper. The filtrate was the evaporated in a vacuole to produce an ethanol extract (277 g). The ethanol extract was suspended in distilled water (500 ml) and then filtered. The residue derived from the filtration was dissolved in hot ethanol and filtered again. The filtrate was then evaporated in a vacuole to obtain a standardized fraction of S. miltiorrhiza (PF2401-SF, 20 g, 1.0 w/w%). High performance liquid chromatography (HPLC) was used to determine the content of TsI in the standardized fraction as follows (Fig. 1). In a 10-ml volumetric flask, the standard compound (~4 mg) was accurately weighed and dissolved in HPLC-grade methanol to prepare a stock solution. A working calibration solution was prepared with a range from 25 to 400 µg/ml by successive 2-fold serial dilution of the stock solution with methanol. A Sykam 2100 series HPLC system (Sykam, Gilching, Germany) equipped with a column oven, a binary pump, and a degasser was used. A 10-µl volume of standard or sample solution was injected directly into an Inertsil ODS-3 column (4.6 mm×150 mm, 5 µm, GL Sciences, Inc., Tokyo, Japan) using a mixture of acetonitrile-water (65:35, v/v). The flow rate was 1.0 ml/min, and detection was carried out at UV 254 nm.
Eight-week-old male C57BL/6 mice (body weight, 20-25 g) obtained from the Experimental Animal Center, Kangwon International University, Chunchon, South Korea were used. The animals were housed in a conventional cage under adequate temperature (23℃) and humidity (60%) conditions, a 12-hour light/12-hour dark cycle, and unlimited access to water and food. Animal handling and care was in line with international laws and policies (National Institutes of Health [NIH] Guide for the Care and Use of Laboratory Animals, NIH Publication no. 85-23, 1985, revised 1996), and the study was approved (approval no. Hallym-1-35) by the Hallym Medical Center Institutional Animal Care and Use Committee (IACUC). We aimed to minimize the number of animals used and avoid animal suffering.
To elucidate the neuroprotective effects of TsI against ischemic damage, the mice were divided into 3 groups: a sham-operated group (sham group); a vehicle-treated ischemia group; and a TsI-treated (10 mg/kg) ischemia group. Vehicle and TsI were administered intraperitoneally 30 minutes before ischemic surgery. TsI was dissolved in dimethyl sulfoxide (DMSO) and diluted to the desired concentration with saline (final DMSO concentration, 1%), and the same dose of DMSO was administered to animals in the vehicle-treated group. TsI dose was selected based on the findings of a previous study [15]. In this experiment, the dose was the minimum required for neuroprotection in the ischemic brain [14].
We used 21 male mice (n=7 per group) in this study. The experimental animals were anesthetized with a mixture of 2.5% isoflurane in 33% oxygen and 67% nitrous oxide. A midline ventral incision was then made in the neck, and the left common carotid artery was isolated, freed of nerve fibers, and permanently ligated with 5-0 surgical silk. Following a 2-hour recovery and feeding period, the animals were exposed to a 30-minute period of hypoxia (92% N2, 8% O2) by placing them in airtight containers partially submerged in a 37℃ water bath to maintain a constant thermal environment.
Neurological deficits in the mice at 1 day after the onset of hypoxia/ischemia were assessed and scored as described in a previous paper [16]. The following scoring was used: 0, no observable neurological deficit (normal); 1, failure to extend left forepaw on the lifting of the body by the tail (mild); 2, circling to the contralateral side (moderate); and 3, leaning to the contralateral side at rest or no spontaneous motor activity (severe). Animals that did not show neurological deficits were excluded from the study.
The mice were euthanized 24 hours after hypoxia-ischemia and perfused transcardially with 0.1-M phosphate-buffered saline (PBS, pH 7.4). Brains were removed, and 1-mm coronal sections were dissected from the frontal pole using a mouse brain slicer (brain Matrix, ASI Instruments, Houston, TX, USA). Six slices were selected according to the mouse brain atlas [17], including the main portion of the infarct. The slices were incubated for 30 minutes in 2% 2,3,5-triphenyltetrazolium chloride (TTC) solution (Sigma-Aldrich Chemical Co., St. Louis, MO, USA) at 37℃, and fixed by immersion in 4% paraformaldehyde solution in PBS (pH 7.4) for 6 hours. The brain tissues were cryoprotected by infiltration with 30% sucrose overnight. To evaluate infarct volume, images of TTC-stained brain sections (n=7 per group) were obtained using a digital camera (Sony, Tokyo, Japan). Measurements were obtained by manually outlining the margins of infarcted areas and were then analyzed using NIH Image software. The infarct volumes were calculated by multiplying the size of the infarcted area by the slice thickness and summing the volume of the 6 slices. Because post-ischemic brain edema would increase brain volume in the infarcted area, the corrected infarct volume was calculated to compensate for the effect of brain edema. An edema index was calculated by dividing the total volume of the hemisphere ipsilateral to hypoxia-ischemia by the total volume of the contralateral hemisphere.
Brain tissues were embedded in paraffin and were then sectioned on a microtome (Leica, Heidelburg, Germany) into 5-µm coronal sections. The sections were mounted on slides and were stained with H&E following standard histochemical procedures. After dehydration, the sections were mounted with Canada Balsam (Kanto, Tokyo, Japan). Images were obtained using a light microscope (BX53, Olympus, Hamburg, Germany) equipped with a digital camera (DP72, Olympus) connected to a PC monitor.
To confirm neuronal death in the brain after hypoxia-ischemia in sham- and ischemia-operated animals, F-J B histofluorescence-a staining procedure using a high-affinity fluorescent marker for localization of neuronal degeneration-was conducted 1 day after hypoxic-ischemic surgery under the same conditions for all the animals. F-J B histofluorescence staining procedures were accomplished according to the methods described in a previous study [18]. In brief, the sections were immersed in a solution containing 1% sodium hydroxide in 80% alcohol, followed by immersion in a solution of 70% alcohol. Next, the sections were transferred to a solution of 0.06% potassium permanganate and then to a 0.0004% F-J C (Histochem, Jefferson, AR, USA) staining solution. After washing, the sections were placed on a slide warmer (approximately 50℃) and examined under an epifluorescent microscope (Carl Zeiss, Oberkochen, Germany) with blue excitation light (450-490 nm) and a barrier filter. In this method, neurons that undergo degeneration fluoresce brightly in comparison with the background [19].
All measurements were obtained to ensure objectivity in blind conditions (2 observers for each experiment), and control and experimental samples were assayed under the same conditions. The number of H&E- and F-J B-positive neurons in a 250×250-µm square were counted in a location around the penumbra and core region of the cerebral cortex using an image analyzing system (Optimas 6.5, CyberMetrics, Scottsdale, AZ, USA). According to anatomical landmarks corresponding to AP +1.1 to -0.6 mm of the mouse brain atlas [17], the studied tissue sections were selected with 300-µm intervals. Cell counts were obtained by averaging the total number of H&E- and F-J B-positive neurons from each animal per group, and a ratio of the count was calibrated as a percentage.
Compared with vehicle treatment, treatment with TsI (10 mg/kg) significantly reduced neurological deficits and increased functional recovery from hypoxia-ischemia at 1 day after hypoxia (Fig. 2).
The neuroprotective effect of TsI was examined 1 day after hypoxia-ischemia, and the infarct volume of brain sections stained with TTC was measured using computer-assisted image analysis (Fig. 3). Infarction was not detected in any regions of the brain in the sham group (Fig. 3A, D). In the vehicle-treated hypoxia-ischemia group, infarct area was clearly observed, and the mean infarct volume was 37.86±3.2 mm3 as measured with TTC staining (Fig. 3B, D). However, in the TsI-treated hypoxia-ischemia group, the mean infarct volume was much smaller (11.65±2.7 mm3) than that in the vehicle-treated hypoxia-ischemia group (Fig. 3C, D).
As shown in Fig. 4, the ischemic lesion was easily discernible in undamaged areas in the vehicle-treated hypoxia-ischemia group. In the contralateral region, no significant change in the number and morphology of H&E-positive cells was found in all the groups (Fig. 4A, C, E). In the ipsilateral region, normally H&E stained cells were detected in the sham group (Fig. 4B, G), and most H&E-positive cells in the vehicle-treated hypoxia-ischemia group were shrunken with eosinophilic cytoplasm and triangulated pyknotic nuclei, indicating irreversible neuronal injury (Fig. 4D, G). The number of H&E-positive cells in this group was also 60.3% lower than that seen in the sham group (Fig. 4G). However, in the TsI-treated hypoxia-ischemia group, H&E-stained cells were similar to those in the sham group, and degenerating cells were slightly observed in the damaged area; the number of H&E-positive cells was much higher (80.2%) than that in the vehicle-treated hypoxia-ischemia group (Fig. 4F, G).
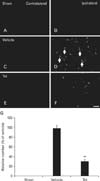
F-J B histofluorescence staining was also performed to confirm the neuronal degeneration in the infarct region one day after the induction of hypoxia-ischemia. F-J B-positive neurons were not detected in any brain regions of the sham group (Fig. 5A, B, G) or in the contralateral region of vehicle- and TsI-treated hypoxia-ischemia groups (Fig. 5C, E). In contrast, in the vehicle-treated hypoxia-ischemia group, many F-J B-positive neurons were observed in the ipsilateral region, (Fig. 5D, G). However, in the TsI-treated hypoxia-ischemia group, the number of F-J B-positive neurons was 34.2% lower than that in the vehicle-treated hypoxia-ischemia group (Fig. 5F, G).
Danshen has been widely used in the Republic of Korea, China, Japan, United States, and Europe for the treatment of vascular diseases such as hypertension, stroke, hyperlipidemia, and atherosclerosis [20-22]. The beneficial attributes of Danshen include the ability to dilate coronary arteries, increase coronary blood flow, scavenge free radicals in ischemic insults, and reduce cellular damage induced by ischemia [7]. Clinical trials indicate that Danshen may have beneficial effects in patients with angina pectoris, acute myocardial ischemia, and stroke [23]. This mechanism may involve the ability of Danshen to enhance antioxidant defense enzyme activities and decrease the production of oxygen-derived free radicals [24]. In addition to this, tanshinones (the major lipophilic components extracted from Danshen) have been shown to have an anti-ischemic stroke effect. Tanshinones have a strong inhibitory effect on inflammatory responses in rats with myocardial infarction [25]. They also have a neuroprotective effect on both permanent and transient focal cerebral ischemia in mice [10, 11]. Among the tanshinones, TsIIA has protective effects against hypertension induced by angiotensin II in cultured cardiac cells [26] and reduces lipid peroxidation induced by adriamycin in mice [27]. TsI has been clarified over the several decades. Treatment with TsI restored cardiac contractile force in an isolated heart undergoing hypoxia and reoxygenation, which was associated with ATP metabolism in the myocardium [28]. In addition, TsI attenuates production of inflammatory cytokines such as interleukin-12 and interferon-γ in immune cells [29].
This is the first study to report that in a mouse model of hypoxia-ischemia, mice treated with TsI showed a significant reduction in both infarct volume and histopathological change in the damaged brain region compared to that in vehicle-treated hypoxia-ischemia mice. The mouse model of hypoxia-ischemia used in this study was originally developed in adult rats [30], and the model has been used almost exclusively in newborn and immature rodents [31, 32] for the past 2 decades. Here, we used adult mice and implemented the model in 2 parts: unilateral transection of 1 common carotid artery followed by exposure to 8% oxygen. This model is different from a gerbil model of transient cerebral ischemia that is produced through bilateral occlusion of the carotid arteries. The Mongolian gerbil is considered to be a good animal model for investigating the mechanisms of selective neuronal death following transient global cerebral ischemia [33]. The most distinctive difference between these 2 models is the brain region and time of cell death. In the mouse model, cell death starts in the striatum from 1 day after hypoxia-ischemia occurs; however, in the gerbil model of transient cerebral ischemia, cell death occurs mainly in the hippocampal CA1 region 4 days after transient ischemia. In fact, neuronal death in the CA1 region is known as "delayed neuronal death (DND)" because it occurs very slowly [34].
In the present study, we examined the neuroprotective effects of TsI against ischemic damage using TTC staining for evaluating hypoxia-ischemic infarct, H&E staining for surviving neurons, and F-J B histofluorescence for degenerating neurons.
TTC staining has been used to identify and quantify infarcts in ischemic brain since the 1950s. This staining method is a useful tool for assessing the infarct area because the usefulness of TTC staining depends on its intracellular interaction with respiratory enzymes such as lactate dehydrogenases [35]. In the present study, we found that infarct areas in mice subjected to 30 minutes of hypoxia-ischemia can be visualized and quantified using TTC staining (Fig. 3). TTC staining revealed infarction in the ipsilateral region, which appears to be a particularly susceptible area. However, treatment with TsI significantly reduced the infarction area. These results indicate that TsI therapy dramatically decreases infarct volume.
H&E staining has been widely used as a histological method for identifying cell damage in the nervous system, because the stain binds to acidic components in the cytoplasm [36, 37]. Damaged cells show various features, including a shrunken cell body with pyknosis and chromatolysis [37]. In this study, H&E staining revealed cellular degeneration in the ipsilateral region after 30 minutes of hypoxia-ischemia. Despite being exposed to 30 minutes of hypoxic conditions, mice treated with TsI had significantly reduced cellular degeneration in the infarct region. In addition, the results of H&E staining were consistent with the damaged area identified by TTC staining. This finding is supported by our previous study that showed that TsI derived from Danshen had the strongest neuroprotective effect against transient cerebral ischemic damage in gerbils [15].
Degenerating neurons tend to be hyperchromic under H&E staining [36]. Damaged cells show various features, including a shrunken cell body with pyknosis and chromatolysis [37]. However, this method is insufficient to discriminate neuronal degeneration, because argyrophilic dark neurons, which are damaged by insults, would ultimately result in dying or recovering neurons [38]. Therefore, in the present study, we used F-J B histofluorescence to elucidate the degree of neuronal damage after hypoxia-ischemia. F-J B has a good affinity for entirely degenerated neurons (cell bodies, dendrites, axons, and axon terminals), and is a useful marker to study neuronal degeneration after ischemic injury [19]. In the present study, neurons with ischemic damage were well detected in the ipsilateral region after hypoxia-ischemia.
Although many investigators have focused on the protective effect of diterpenoids in Danshen extract against brain damage, including ischemia, few studies have assessed the neuroprotective effect of TsIIA and/or TsIIB in a mouse model of transient focal cerebral ischemia [10] or rat models of transient focal/global cerebral ischemia [12-14]. However, in the present study, we could not demonstrate the pharmacological properties of TsI. Therefore, further studies are needed to investigate the pharmacological properties of TsI so that its neuroprotective effects may be used in clinical medicine.
In conclusion, TsI has a very strong neuroprotective effect against hypoxic-ischemic injury, and TsI treatment may be beneficial in hypoxic-ischemic patients.
Figures and Tables
Fig. 2
Neurological deficits observed in the sham, vehicle-treated, and tanshinone I (TsI)-treated hypoxia-ischemia groups 24 hours after induction of hypoxia-ischemia. The TsI-treated hypoxia-ischemia group showed a significant reduction in neurological deficits (n=7 per group; **P<0.05, significantly different from the vehicle-treated hypoxia-ischemia group). The bars indicate mean±SEM.

Fig. 3
TTC staining of mouse brain slices of the sham (A), vehicle-treated (B), and tanshinone I (TsI)-treated (C) hypoxia-ischemia groups 24 hours after induction of hypoxia-ischemia. The vehicle-treated hypoxia-ischemia group shows severe infarction (unstained region, *); the TsI-treated hypoxia-ischemia group shows a significant reduction in infarction (arrow). Scale bar in (C)=5 mm (A-C). (D) Infarct volume (mm3) of the sham, vehicle- and TsI-treated hypoxia-ischemia groups 24 hours after hypoxia-ischemia. (n=7 per group; **P<0.05, significantly different from the vehicle-treated hypoxia-ischemia group). The bars indicate mean±SEM.
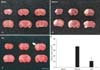
Fig. 4
Hematoxylin and eosin (H&E) staining in the contralateral (A, C, E) and ipsilateral (B, D, F) regions of the cerebral cortex of the sham (A, B), vehicle-treated (C, D), and tanshinone I (TsI)-treated (E, F) hypoxia-ischemia groups 24 hours after induction of hypoxia-ischemia. Many cells (arrows) in the ipsilateral region show eosinophilic cytoplasm in the vehicle-treated hypoxia-ischemia group; H&E-positive neurons in the TsI-treated hypoxia-ischemia group are similar to those in the sham group. Scale bar in (F)=50 µm (A-F). (G) Relative numeric analysis of H&E-positive cells in the ipsilateral region in the sham, vehicle-treated, and TsI-treated hypoxia-ischemia groups (n=7 per group; *P<0.05, significantly different from the sham group; **P<0.05, significantly different from the vehicle-treated hypoxia-ischemia group). The bars indicate mean±SEM.
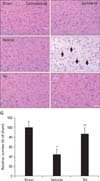
Fig. 5
Fluoro-Jade B (F-J B) histofluorescence staining in the contralateral (A, C, E) and ipsilateral (B, D, F) regions of the cerebral cortex of the sham (A, B), vehicle-treated (C, D), and tanshinone I (TsI)-treated (E, F) groups. Many F-J B-positive cells (arrows) in the ipsilateral region are observed in the vehicle-treated group; a few F-J B-positive cells are observed in the TsI-treated group. Scale bar in (F)=50 µm (A-F). (G) Relative numeric analysis of F-J B-positive cells in the ipsilateral region in the sham, vehicle-treated, and TsI-treated hypoxia-ischemia groups (n=7 per group; **P<0.05, significantly different from the vehicle-treated hypoxia-ischemia group). The bars indicate mean±SEM.
Acknowledgements
The authors would like to thank Mr. Seung Uk Lee for his technical help in this study. This research was supported by the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010-0010580), and by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NO2011-0022812).
References
1. Bei W, Peng W, Ma Y, Xu A. Flavonoids from the leaves of Diospyros kaki reduce hydrogen peroxide-induced injury of NG108-15 cells. Life Sci. 2005; 76:1975–1988.
2. Xu D, Du W, Zhao L, Davey AK, Wang J. The neuroprotective effects of isosteviol against focal cerebral ischemia injury induced by middle cerebral artery occlusion in rats. Planta Med. 2008; 74:816–821.
3. Zeng X, Zhang S, Zhang L, Zhang K, Zheng X. A study of the neuroprotective effect of the phenolic glucoside gastrodin during cerebral ischemia in vivo and in vitro. Planta Med. 2006; 72:1359–1365.
4. Dekanski D, Selaković V, Piperski V, Radulović Z, Korenić A, Radenović L. Protective effect of olive leaf extract on hippocampal injury induced by transient global cerebral ischemia and reperfusion in Mongolian gerbils. Phytomedicine. 2011; 18:1137–1143.
5. Shri R, Singh Bora K. Neuroprotective effect of methanolic extracts of Allium cepa on ischemia and reperfusion-induced cerebral injury. Fitoterapia. 2008; 79:86–96.
6. Zhou L, Zuo Z, Chow MS. Danshen: an overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J Clin Pharmacol. 2005; 45:1345–1359.
7. Han JY, Fan JY, Horie Y, Miura S, Cui DH, Ishii H, Hibi T, Tsuneki H, Kimura I. Ameliorating effects of compounds derived from Salvia miltiorrhiza root extract on microcirculatory disturbance and target organ injury by ischemia and reperfusion. Pharmacol Ther. 2008; 117:280–295.
8. Gu M, Zhang G, Su Z, Ouyang F. Identification of major active constituents in the fingerprint of Salvia miltiorrhiza Bunge developed by high-speed counter-current chromatography. J Chromatogr A. 2004; 1041:239–243.
9. Ren Y, Houghton PJ, Hider RC, Howes MJ. Novel diterpenoid acetylcholinesterase inhibitors from Salvia miltiorhiza. Planta Med. 2004; 70:201–204.
10. Lam BY, Lo AC, Sun X, Luo HW, Chung SK, Sucher NJ. Neuroprotective effects of tanshinones in transient focal cerebral ischemia in mice. Phytomedicine. 2003; 10:286–291.
11. Dong K, Xu W, Yang J, Qiao H, Wu L. Neuroprotective effects of Tanshinone IIA on permanent focal cerebral ischemia in mice. Phytother Res. 2009; 23:608–613.
12. Chen Y, Wu X, Yu S, Fauzee NJ, Wu J, Li L, Zhao J, Zhao Y. Neuroprotective capabilities of tanshinone IIA against cerebral ischemia/reperfusion injury via anti-apoptotic pathway in rats. Biol Pharm Bull. 2012; 35:164–170.
13. Hei M, Luo Y, Zhang X, Liu F. Tanshinone IIa alleviates the biochemical changes associated with hypoxic ischemic brain damage in a rat model. Phytother Res. 2011; 25:1865–1869.
14. Tang C, Xue H, Bai C, Fu R, Wu A. The effects of tanshinone IIA on blood-brain barrier and brain edema after transient middle cerebral artery occlusion in rats. Phytomedicine. 2010; 17:1145–1149.
15. Park OK, Choi JH, Park JH, Kim IH, Yan BC, Ahn JH, Kwon SH, Lee JC, Kim YS, Kim M, Kang IJ, Kim JD, Lee YL, Won MH. Comparison of neuroprotective effects of five major lipophilic diterpenoids from Danshen extract against experimentally induced transient cerebral ischemic damage. Fitoterapia. 2012; 83:1666–1674.
16. Huang Z, Huang PL, Panahian N, Dalkara T, Fishman MC, Moskowitz MA. Effects of cerebral ischemia in mice deficient in neuronal nitric oxide synthase. Science. 1994; 265:1883–1885.
17. Franklin KB, Paxinos G. The mouse brain in stereotaxic coordinates. San Diego: Academic Press;1997.
18. Candelario-Jalil E, Alvarez D, Merino N, Leon OS. Delayed treatment with nimesulide reduces measures of oxidative stress following global ischemic brain injury in gerbils. Neurosci Res. 2003; 47:245–253.
19. Schmued LC, Hopkins KJ. Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000; 874:123–130.
20. Ji XY, Tan BK, Zhu YZ. Salvia miltiorrhiza and ischemic diseases. Acta Pharmacol Sin. 2000; 21:1089–1094.
21. Chan K, Chui SH, Wong DY, Ha WY, Chan CL, Wong RN. Protective effects of Danshensu from the aqueous extract of Salvia miltiorrhiza (Danshen) against homocysteine-induced endothelial dysfunction. Life Sci. 2004; 75:3157–3171.
22. Zhang Y, Li X, Wang Z. Antioxidant activities of leaf extract of Salvia miltiorrhiza Bunge and related phenolic constituents. Food Chem Toxicol. 2010; 48:2656–2662.
23. Sun J, Huang SH, Tan BK, Whiteman M, Zhu YC, Wu YJ, Ng Y, Duan W, Zhu YZ. Effects of purified herbal extract of Salvia miltiorrhiza on ischemic rat myocardium after acute myocardial infarction. Life Sci. 2005; 76:2849–2860.
24. Lam FF, Yeung JH, Chan KM, Or PM. Relaxant effects of Danshen aqueous extract and its constituent Danshensu on rat coronary artery are mediated by inhibition of calcium channels. Vascul Pharmacol. 2007; 46:271–277.
25. Ren ZH, Tong YH, Xu W, Ma J, Chen Y. Tanshinone II A attenuates inflammatory responses of rats with myocardial infarction by reducing MCP-1 expression. Phytomedicine. 2010; 17:212–218.
26. Takahashi K, Ouyang X, Komatsu K, Nakamura N, Hattori M, Baba A, Azuma J. Sodium tanshinone IIA sulfonate derived from Danshen (Salvia miltiorrhiza) attenuates hypertrophy induced by angiotensin II in cultured neonatal rat cardiac cells. Biochem Pharmacol. 2002; 64:745–749.
27. Zhou GY, Zhao BL, Hou JW, Ma GE, Xin WJ. Protective effects of sodium tanshinone IIA sulphonate against adriamycin-induced lipid peroxidation in mice hearts in vivo and in vitro. Pharmacol Res. 1999; 40:487–491.
28. Yagi A, Fujimoto K, Tanonaka K, Hirai K, Takeo S. Possible active components of tan-shen (Salvia miltiorrhiza) for protection of the myocardium against ischemia-induced derangements. Planta Med. 1989; 55:51–54.
29. Kang BY, Chung SW, Kim SH, Ryu SY, Kim TS. Inhibition of interleukin-12 and interferon-gamma production in immune cells by tanshinones from Salvia miltiorrhiza. Immunopharmacology. 2000; 49:355–361.
30. Levine S. Anoxic-ischemic encephalopathy in rats. Am J Pathol. 1960; 36:1–17.
31. Rice JE 3rd, Vannucci RC, Brierley JB. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol. 1981; 9:131–141.
32. Vannucci RC, Connor JR, Mauger DT, Palmer C, Smith MB, Towfighi J, Vannucci SJ. Rat model of perinatal hypoxic-ischemic brain damage. J Neurosci Res. 1999; 55:158–163.
33. Zhang YB, Kan MY, Yang ZH, Ding WL, Yi J, Chen HZ, Lu Y. Neuroprotective effects of N-stearoyltyrosine on transient global cerebral ischemia in gerbils. Brain Res. 2009; 1287:146–156.
34. Kirino T, Sano K. Selective vulnerability in the gerbil hippocampus following transient ischemia. Acta Neuropathol. 1984; 62:201–208.
35. Xue QS, Yu BW, Wang ZJ, Chen HZ. Effects of ketamine, midazolam, thiopental, and propofol on brain ischemia injury in rat cerebral cortical slices. Acta Pharmacol Sin. 2004; 25:115–120.
36. Stensaas SS, Edwards CQ, Stensaas LJ. An experimental study of hyperchromic nerve cells in the cerebral cortex. Exp Neurol. 1972; 36:472–487.
37. Bartus RT, Dean RL, Cavanaugh K, Eveleth D, Carriero DL, Lynch G. Time-related neuronal changes following middle cerebral artery occlusion: implications for therapeutic intervention and the role of calpain. J Cereb Blood Flow Metab. 1995; 15:969–979.
38. Gallyas F, Zoltay G, Dames W. Formation of "dark" (argyrophilic) neurons of various origin proceeds with a common mechanism of biophysical nature (a novel hypothesis). Acta Neuropathol. 1992; 83:504–509.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download