Abstract
Women with tubal ectopic pregnancies have high levels of circulating interleukin 6 (IL-6). IL-6 treatment in vitro significantly reduces the ciliary activity of tubal epithelium. The effects of IL-6 on target cells occur via the formation of a high-affinity complex with its receptors IL-6Rα and glycoprotein 130 (Gp130). IL-6Rα is specifically expressed in the cilia of the epithelial cells. In this study, we performed a quantitative reverse transcriptase polymerase chain reaction to determine the mRNA expression of IL-6Rα and Gp130 in the fallopian tubes obtained from 12 women with ectopic pregnancies, 12 women with normal pregnancies, and 12 healthy nonpregnant women in the luteal phase of their menstrual cycle. Fallopian tubes were evaluated from specimens taken during tubal ligation in normal pregnancies and nonpregnant fertile women or during tubal surgery in ectopic pregnancies. We observed that IL-6Rα mRNA expression in fallopian tubes was increased in ectopic pregnancy compared with that in the midluteal phase. We also found that the Gp130 mRNA expression was significantly lower in fallopian tubes from ectopic pregnancies than in those from nonpregnant women during the midluteal phase of their menstrual cycle, although its expression was noticeably high in fallopian tubes in the midluteal phase, which suggests that high Gp130 levels may possibly contribute to embryo transport into the uterus.
Ectopic pregnancy caused by tubal rupture is one of the main reasons for maternal morbidity during the first trimester [1]. Ectopic pregnancy occurs in 2-3 of every 100 pregnancies [2]. The ampullary region of the fallopian tube is the most common location for an ectopic pregnancy with a rate of 70% [2, 3]. Although methotrexate is used for the clinical treatment of ectopic pregnancy, most ectopic pregnancies are treated by laparoscopic surgery or, in more serious cases, by open abdominal surgery [4]. Women with a history tubalectopic pregnancy have an increased rate of infertility and/or future tubal ectopic pregnancy [4-6].
Tubal implantation and the development of ectopic pregnancies are characterized by a failure in tubal transportation mechanisms and receptive phenotype in the fallopian tube [7]. Available studies indicate that embryo-tubal transportation is achieved through a complicated interaction between muscle contractions, ciliary beating, and the flow of tubal secretions [5, 8]. Tubal damage caused by infections and smoking leads to the development of a proinflammatory phenotype in the fallopian tubes. To be more specific, this proinflammatory condition is caused by the upregulation of cytokines that promote embryo receptivity and consequently, lead to ectopic pregnancy.
Interleukin 6 (IL-6) has physiological roles in reproduction through the regulation of ovarian steroid production, folliculogenesis, and in the early events associated with implantation [9-12]. Proinflammatory and immunoregulatory cytokine levels are very high in endometriosis and pelvic inflammatory diseases, which are known risk factors for ectopic pregnancy [13].
IL-6 treatment in vitro significantly reduces the ciliary activity of tubal epithelium, whereas anti-IL-6 restores ciliary activity [13]. Clinical studies show that women with tubal ectopic pregnancies have high circulating IL-6 levels [6]. In other studies, IL-6 expression significantly increased near the implantation site in fallopian tubes with ectopic pregnancy as compared to that in normal fallopian tubes [14]. IL-6 signaling may be important for gamete and embryo transportation, whereas abnormal IL-6 levels are thought to alter tubal transport [15].
The effects of IL-6 on target cells occur via the formation of a high-affinity complex composed of 80-kDa IL-6, glycoprotein 80 (Gp80) (α-chain, IL-6 receptor α [IL-6Rα]), and 130-kDa signal transducer Gp130 (β-chain) [16]. IL-6Rα is specifically expressed in the cilia of the epithelial cells [15].
A better understanding of the mechanism by which an embryo implants in the fallopian tube instead of the uterus may lead to improved methods for early diagnosis of ectopic pregnancy or approaches to prevent ectopic implantation in women with a history of ectopic pregnancy [17]. As such, the current study aimed at analyzing the IL-6Rα and Gp130 expression in detail in the fallopian tubes of women with ectopic pregnancy.
Ethical approval for this study was obtained from the Ethics Committee of Shahid Beheshti University of Medical Sciences. All participants signed informed consent letters. Specimens from the ampullary region of the fallopian tubes were obtained from all 36 participants. The case group consisted of 12 patients (age, 22-35 years) who had undergone salpingectomy for tubal pregnancy. All participants in this group had spontaneous pregnancies. They did not receive any exogenous hormone treatment or use any intrauterine devices within the last 3 months before the surgery. Gestational age in this study was determined by the date of commencement of the last menstrual period within 6-9 weeks. The first control group consisted of 12 premenopausal patients (age, 35-44 years) who had undergone hysterectomy and bilateral salpingo-oophorectomy for benign disease that did not affect the fallopian tubes. Histological dating was performed according to the criteria of Noyes et al. [18] to confirm the luteal phase of the menstrual cycle. They had a history of normal intrauterine pregnancy, regular menses, and a clear memory of their last menstrual period. They did not receive any exogenous hormone treatment. The last group consisted of 12 patients (age, 32-38 years) who had undergone postpartum tubal ligation during cesarean delivery (>37 weeks gestation).
For the case group, the fallopian tubes were excised at least 1 cm away from the implantation site to avoid collecting any embryonic or trophoblastic tissue and to ensure the integrity of tubal morphology and function [19]. The mucosal layers of the fallopian tubes of all 36 patients were dissected macroscopically [20] and directly immersed in RNAlater (Ambion, Qiagen, Austin, TX, USA) at 4℃ overnight and then flash-frozen at -20℃ for RNA extraction.
Total RNA was isolated usingIsol RNA lysis reagent (5PRIME, Gaithersburg, MD, USA), according to the manufacturer's instructions. The RNA concentration was determined by spectrophotometric analysis (Eppendorf, Hamburg, Germany) and agarose gel electrophoresis.
RNA (10 µL) was reverse transcribed into cDNA using a 10-µL reaction with random hexamers and the REVERTA-L RT reagents kit (containing RT-G-mix-1, RT-mix-1, and MMlv; AmpliSens, Moscow, Russia). Quantitative RT-PCR measures the relative expression of the genes of interest, which was assessed by quantitative PCR on a Rotor-Gene 6000 Series Software instrument (Corbett, Belgium) using 5× HOT FIREPol EvaGreenqPCR Mix Plus (Solis BioDyne, Tartu, Estonia) and primers from Metabion (Martinsried, Germany) (Table 1). Primers had been previously checked by conventional RT-PCR and electrophoresis on 1.5% agarose gel (Fig. 1). Each well of the PCR plate contained 4 µL of EvaGreen, 0.5 µL of forward primer, 0.5 µL of reverse primer, 13 µL of water, and 2 µL of cDNA. The amplification was performed as follows: step 1, initial denaturation (1 cycle of incubation at 95℃ for 15 minutes); step 2, denaturation (40 cycles of incubation at 95℃ for 15 seconds); step 3, annealing (incubation at 65℃ for 20 seconds); and step 4, elongation (incubation at 72℃ for 20 seconds). Amplification of the housekeeping gene glyceraldehyde-3-phosphatedehydrogenase (GAPDH) transcripts was performed simultaneously to confirm RNA integrity, efficiency, and quantification of cDNA. As a negative control for all the reactions, distilled water was used in place of the cDNA. All experiments were performed in duplicate. For the quantitative PCR, the following cycle threshold (Ct) equations were used: ΔCt=Ct (gene of interest)-Ct(housekeeping gene); ΔΔCt=ΔCt (sample)-ΔCt (calibrator); and relative quantity=2ΔΔCt [21]. For quantification, standard curves of serial dilutions extracted from the appropriate purified cDNA were used.
Statistical analyses were performed using the SPSS ver. 20 (SPSS Inc., Chicago, IL, USA). Significant differences were determined using one-way analysis of variance and Tukey's post hoc analysis to compare the fallopian tubes obtained from the three groups of participants. P<0.05 was considered statistically significant. Excel was used for calculations.
We measured IL-6Rα and Gp130 mRNA levels in the fallopian tubes of women with ectopic pregnancies, normal pregnant women, and nonpregnant women in the luteal phase of their menstrual cycle.
Expression of IL-6Rα mRNA was lower in the fallopian tubes of women with ectopic pregnancies than in those of normal pregnant women. However, the difference was not significant (P>0.05).
In fallopian tubes of women with ectopic pregnancies, IL-6Rα mRNA expression was significantly higher than that in the fallopian tubes of nonpregnant women during the luteal phase (P<0.05) (Fig. 2).
We also observed that the Gp130 mRNA expression was significantly higher in the fallopian tubes of nonpregnant women than in the other groups (P<0.05). Expression of Gp130 mRNA was significantly lower in the fallopian tubes of women with ectopic pregnancies than in those of the nonpregnant women (P<0.05) (Fig. 3).
In addition, the Gp130 mRNA expression was higher in the women with ectopic pregnancies than in the women with normal pregnancies, although this difference was not statistically significant (P>0.05).
This is the first study to compare the IL-6Rα and Gp130 mRNA expression in the fallopian tubes of women with ectopic pregnancies with that in the fallopian tubes of nonpregnant women and women with normal pregnancies. We identified the existence of IL-6Rα in fallopian tube cells. This finding is in agreement with studies reporting that IL-6Rα is specifically expressed in the cilia of human fallopian tubes in vivo, suggesting that this receptor contributes to the functions specific to ciliated epithelial cells, including ciliary beating, which is coordinated with tubal peristalsis [22, 23]. Ciliary beating are regarded as the principal factors responsible for propelling the gametes and embryo through the fallopian tube, even when muscular activity is blocked. In other words, ciliary beating frequency can be modulated by IL-6 in vitro [13].
Women with tubal ectopic pregnancies have especially high serum IL-6 levels compared to women with normal pregnancies [24, 25]. Increased proinflammatory cytokine levels, which are induced by paracrine signaling of the embryo, characterize the endometrium during early implantation [14, 26]. As such, it is believed that signal upregulation of the proteins is required for embryo receptivity, adhesion, and trophoblast invasion [27-30]. For this reason, cytokines are known to be pivotal in the interaction between the fallopian tube and the developing embryo [29, 30]. A recent study has also shown that ectopic pregnancy is associated with a significant increase in the expression of IL-6, IL-8, and CXCR1 [14].
The current findings indicate that the IL-6Rα mRNA expression in the fallopian tubes of women with tubal pregnancy is higher than that in the fallopian tubes of nonpregnant women. Cilia-localized IL-6Rα is a target of estrogen regulation in mouse and human fallopian tubes [15]. More specifically, estrogen selectively downregulates IL-6Rα expression via estrogen receptor alpha (ERα), which induces the estrogensignal that influences IL-6Rα expression in mouse fallopian tubes. In addition, a previous study also showed that circulating estrogen levels are higher in women with tubal ectopic pregnancy than in nonpregnant women [31, 32]. Because ERα is frequently lost in the implantation and nonimplantation sites [33] of the fallopian tubes in women who have had an ectopic pregnancy [34, 35], we suggest that changes in ERα expression may cause dysfunction of IL-6Rα expression [33], which consequently leads to an increased possibility of tubal ectopic pregnancy.
Results from a recent study, in contrast to ours, has reported that IL-6Rα expression in the fallopian tubes of women with ectopic pregnancies was not different from that in the fallopian tubes of the normal group [14]. The differences in results may be explained by the use of different methods and the lack of fresh tissue in the above mentioned study.
We also found that the Gp130 mRNA expression was significantly lower in the fallopian tubes of women with ectopic pregnancies than that in the fallopian tubes of nonpregnant women. However, to our knowledge, no other studies have been conducted on Gp130 mRNA expression in ectopic pregnancy to compare with our study. One study conducted on Gp130 located in the epithelium of the mouse fallopian tube reported that regulation of Gp130 expression is independent of ovarian steroid hormones, whereas IL-6Rα expression is dependent on ovarian steroid hormones [15]. In agreement with this study, our study seems to reveal that Gp130 mRNA expression, in contrast to IL-6Rα mRNA expression, is significantly lower in the fallopian tubes of women with ectopic pregnancies than in the fallopian tubes of nonpregnant women. Another probability for the observed difference in expression of the two receptors is that Gp130 can bind with other cytokines as well as IL-6, which is known to be produced by the human embryo [35, 36].
In addition, Gp130 expression in fallopian tubes in the luteal phase was found to be noticeably high, suggesting that high Gp130 levels may contribute to embryo transport into the uterus.
In summary, we have found that IL-6Rα and Gp130 are expressed in the epithelium of human fallopian tubes. In addition, IL-6Rα expression in the fallopian tubes of women with tubal pregnancy was found to be higher than that in the fallopian tubes of nonpregnant women. Our results also indicated that Gp130 expression is lower in the fallopian tubes of women with ectopic pregnancy than in the fallopian tubes of nonpregnant women.
The physiological relevance of our findings must be interpreted with caution because differences in IL-6Rα and Gp130 mRNA expression between the fallopian tubes from ectopic pregnancies and those from late pregnancy were not statistically significant. However, it was not possible to compare normal fallopian tubes of women at a comparable gestational age. Additional studies are required to quantify these findings at the protein level.
Figures and Tables
Fig. 1
Reverse transcription-polymerase chain reaction with IL-6Rα (251 base pairs), Gp130 (326 base pairs), and GAPDH (98 base pairs) primers and electrophoresis on 1.5% agarose gel. IL-6Rα, interleukin 6 receptor α; Gp130, glycoprotein 130; GAPDH, glyceraldehyde-3-phosphatedehydrogenase.
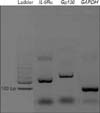
Fig. 2
Interleukin 6 receptor α (IL-6Rα) expression in the fallopian tube. IL-6Rα mRNA expression was higher in the fallopian tubes of women with ectopic pregnancies than in the fallopian tubes of the nonpregnant women in the luteal phase of their menstrual cycle (control group). Expression of IL-6Rα mRNA was lower in the fallopian tubes of women with ectopic pregnancies than in the fallopian tubes of normal pregnant women, but this difference was not significant (P>0.05). *indicates a significant difference from the control group (P<0.05).

Fig. 3
Glycoprotein 130 (Gp130) expression in the fallopian tube. Expression of Gp130 mRNA was lower in the fallopian tubes of women with ectopic pregnancies than in the fallopian tubes of nonpregnant woman in the luteal phase of their menstrual cycle (control group). Expression of Gp130 mRNA was higher in the women with ectopic pregnancies than in normal pregnant women, although this difference was not statistically significant (P>0.05). *indicates a significant difference from the control group (P<0.05).

Acknowledgements
This research was supported by Shahid Beheshti University of Medical Sciences (Grants 10164).
References
1. Kriebs JM, Fahey JO. Ectopic pregnancy. J Midwifery Womens Health. 2006; 51:431–439.
2. Bouyer J, Coste J, Fernandez H, Pouly JL, Job-Spira N. Sites of ectopic pregnancy: a 10 year population-based study of 1800 cases. Hum Reprod. 2002; 17:3224–3230.
3. Kiran G, Kiran H, Ertopcu K, Kilinc M, Ekerbicer HC, Vardar MA. Tuba uterina leukemia inhibitory factor concentration does not increase in tubal pregnancy: a preliminary study. Fertil Steril. 2005; 83:484–486.
4. Shao R, Zou S, Wang X, Feng Y, Brännström M, Stener-Victorin E, Billig H. Revealing the hidden mechanisms of smoke-induced fallopian tubal implantation. Biol Reprod. 2012; 86:131.
5. Shao R. Understanding the mechanisms of human tubal ectopic pregnancies: new evidence from knockout mouse models. Hum Reprod. 2010; 25:584–587.
6. Farquhar CM. Ectopic pregnancy. Lancet. 2005; 366:583–591.
7. Shaw JL, Horne AW. The paracrinology of tubal ectopic pregnancy. Mol Cell Endocrinol. 2012; 358:216–222.
8. Lyons RA, Saridogan E, Djahanbakhch O. The reproductive significance of human Fallopian tube cilia. Hum Reprod Update. 2006; 12:363–372.
9. Orsi NM, Tribe RM. Cytokine networks and the regulation of uterine function in pregnancy and parturition. J Neuroendocrinol. 2008; 20:462–469.
10. Dimitriadis E, White CA, Jones RL, Salamonsen LA. Cytokines, chemokines and growth factors in endometrium related to implantation. Hum Reprod Update. 2005; 11:613–630.
11. Pfeilschifter J, Köditz R, Pfohl M, Schatz H. Changes in proinflammatory cytokine activity after menopause. Endocr Rev. 2002; 23:90–119.
12. Machelon V, Emilie D, Lefevre A, Nome F, Durand-Gasselin I, Testart J. Interleukin-6 biosynthesis in human preovulatory follicles: some of its potential roles at ovulation. J Clin Endocrinol Metab. 1994; 79:633–642.
13. Papathanasiou A, Djahanbakhch O, Saridogan E, Lyons RA. The effect of interleukin-6 on ciliary beat frequency in the human fallopian tube. Fertil Steril. 2008; 90:391–394.
14. Balasubramaniam ES, Van Noorden S, El-Bahrawy M. The expression of interleukin (IL)-6, IL-8, and their receptors in fallopian tubes with ectopic tubal gestation. Fertil Steril. 2012; 98:898–904.
15. Shao R, Nutu M, Karlsson-Lindahl L, Benrick A, Weijdegård B, Lager S, Egecioglu E, Fernandez-Rodriguez J, Gemzell-Danielsson K, Ohlsson C, Jansson JO, Billig H. Downregulation of cilia-localized Il-6R alpha by 17beta-estradiol in mouse and human fallopian tubes. Am J Physiol Cell Physiol. 2009; 297:C140–C151.
16. Lee BS, Choi JY, Cha JH. Expression of ciliary neurotrophic factor and its receptor in experimental obstructive nephropathy. Anat Cell Biol. 2011; 44:85–97.
17. Al-Azemi M, Refaat B, Amer S, Ola B, Chapman N, Ledger W. The expression of inducible nitric oxide synthase in the human fallopian tube during the menstrual cycle and in ectopic pregnancy. Fertil Steril. 2010; 94:833–840.
18. Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Am J Obstet Gynecol. 1975; 122:262–263.
19. Refaat B, Amer S, Ola B, Chapman N, Ledger W. The expression of activin-betaA- and -betaB-subunits, follistatin, and activin type II receptors in fallopian tubes bearing an ectopic pregnancy. J Clin Endocrinol Metab. 2008; 93:293–299.
20. Lam PM, Briton-Jones C, Cheung CK, Leung SW, Cheung LP, Haines C. Increased messenger RNA expression of vascular endothelial growth factor and its receptors in the implantation site of the human oviduct with ectopic gestation. Fertil Steril. 2004; 82:686–690.
21. Zachariades E, Foster H, Goumenou A, Thomas P, Rand-Weaver M, Karteris E. Expression of membrane and nuclear progesterone receptors in two human placental choriocarcinoma cell lines (JEG-3 and BeWo): effects of syncytialization. Int J Mol Med. 2011; 27:767–774.
22. Halbert SA, Tam PY, Blandau RJ. Egg transport in the rabbit oviduct: the roles of cilia and muscle. Science. 1976; 191:1052–1053.
23. Halbert SA, Becker DR, Szal SE. Ovum transport in the rat oviductal ampulla in the absence of muscle contractility. Biol Reprod. 1989; 40:1131–1136.
24. Kishimoto T. Interleukin-6: discovery of a pleiotropic cytokine. Arthritis Res Ther. 2006; 8:Suppl 2. S2.
25. Leese HJ, Tay JI, Reischl J, Downing SJ. Formation of Fallopian tubal fluid: role of a neglected epithelium. Reproduction. 2001; 121:339–346.
26. Vasquez G, Winston RM, Brosens IA. Tubal mucosa and ectopic pregnancy. Br J Obstet Gynaecol. 1983; 90:468–474.
27. Evans J, Catalano RD, Brown P, Sherwin R, Critchley HO, Fazleabas AT, Jabbour HN. Prokineticin 1 mediates fetal-maternal dialogue regulating endometrial leukemia inhibitory factor. FASEB J. 2009; 23:2165–2175.
28. Jabbour HN, Sales KJ, Catalano RD, Norman JE. Inflammatory pathways in female reproductive health and disease. Reproduction. 2009; 138:903–919.
29. Li HZ, Sun X, Stavreus-Evers A, Gemzell-Danielsson K. Effect of mifepristone on the expression of cytokines in the human Fallopian tube. Mol Hum Reprod. 2004; 10:489–493.
30. Senturk LM, Arici A. Leukemia inhibitory factor in human reproduction. Am J Reprod Immunol. 1998; 39:144–151.
31. Shao R, Egecioglu E, Weijdegård B, Kopchick JJ, Fernandez-Rodriguez J, Andersson N, Billig H. Dynamic regulation of estrogen receptor-alpha isoform expression in the mouse fallopian tube: mechanistic insight into estrogen-dependent production and secretion of insulin-like growth factors. Am J Physiol Endocrinol Metab. 2007; 293:E1430–E1442.
32. Shao R, Ljungström K, Weijdegård B, Egecioglu E, Fernandez-Rodriguez J, Zhang FP, Thurin-Kjellberg A, Bergh C, Billig H. Estrogen-induced upregulation of AR expression and enhancement of AR nuclear translocation in mouse fallopian tubes in vivo. Am J Physiol Endocrinol Metab. 2007; 292:E604–E614.
33. Shao R, Feng Y, Zou S, Weijdegård B, Wu G, Brännström M, Billig H. The role of estrogen in the pathophysiology of tubal ectopic pregnancy. Am J Transl Res. 2012; 4:269–278.
34. Srivastava MD, Lippes J, Srivastava BI. Cytokines of the human reproductive tract. Am J Reprod Immunol. 1996; 36:157–166.
35. Wanggren K, Lalitkumar PG, Hambiliki F, Ståbi B, Gemzell-Danielsson K, Stavreus-Evers A. Leukaemia inhibitory factor receptor and gp130 in the human Fallopian tube and endometrium before and after mifepristone treatment and in the human preimplantation embryo. Mol Hum Reprod. 2007; 13:391–397.
36. Austgulen R, Arntzen KJ, Vatten LJ, Kahn J, Sunde A. Detection of cytokines (interleukin-1, interleukin-6, transforming growth factor-beta) and soluble tumour necrosis factor receptors in embryo culture fluids during in-vitro fertilization. Hum Reprod. 1995; 10:171–176.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download