Abstract
We examined pharyngeal nerve courses in paraffin-embedded sagittal sections from 10 human fetuses, at 25-35 weeks of gestation, by using S100 protein immunohistochemical analysis. After diverging from the glossopharyngeal and vagus nerves at the level of the hyoid bone, the pharyngeal nerves entered the constrictor pharyngis medius muscle, then turned upward and ran superiorly and medially through the constrictor pharyngis superior muscle, to reach either the levator veli palatini muscle or the palatopharyngeus muscle. None of the nerves showed a tendency to run along the posterior surface of the pharyngeal muscles. Therefore, the pharyngeal nerve plexus in adults may become established by exposure of the fetal intramuscular nerves to the posterior aspect of the pharyngeal wall because of muscle degeneration and the subsequent rearrangement of the topographical relationship between the muscles that occurs after birth.
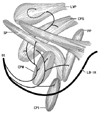
The pharyngeal nerve plexus, or upper pharyngeal nerves, originate from the glossopharyngeal and vagus nerves [1, 2]. According to Ohtsuka et al. [3] and Oda et al. [4], in these nerves, the lingual branches of the glossopharyngeal nerve are notably thick, and they follow a downward course through a slit between the constrictor pharyngis superior and medius muscles (CPS and CPM). Likewise, many atlases of human anatomy (Fig. 1A) [5] display the descending courses of the other, thinner, pharyngeal nerves along the posterior surface of the CPS and CPM. In contrast, Shimokawa et al. [6] demonstrated recurrent upward courses of the pharyngeal nerves to the CPS and levator veli palatini muscle (LVP), along the posterior surface (Fig. 1B). The primary aim of the present study was to confirm the courses of the pharyngeal nerve after divergence of the lingual branches of the glossopharyngeal nerve.
In most textbooks, descriptions of the fetal development of the pharynx concentrate on the development of the mucosal wall from the endoderm [7-9]. Detailed descriptions of the fetal pharyngeal nerves are relatively limited; Doménech-Ratto [10] provided several photographs of the pharyngeal nerves supplying the CPS and CPM in human fetuses with a crown-rump length (CRL) of 20-89 mm. However, in these high-magnification photos, it was difficult to identify the topographical anatomy of the nerve courses. Our recent immunohistochemical study using human fetuses [11] revealed that the pharyngeal muscles are so large at 20-25 weeks of gestation, that the nerves supplying them are clearly identifiable at lower magnifications. Moreover, the fetal upper pharyngeal muscles provide a thick muscle mass, in contrast to the thin, lower pharyngeal wall. Thus, a topographical change between the upper muscles seems to occur in order to form the wall-like configuration in adults. Fetal nerve configurations in the mass-like, upper pharyngeal wall are also likely to be different from the mature adult morphology. Consequently, the secondary aim of this study was to describe the topographical relationships between nerves and muscles in the fetal pharyngeal wall.
This study was performed in accordance with the provisions of the Declaration of Helsinki 1995 (as revised in Edinburgh 2000). We observed the paraffin-embedded histology (sagittal sections) of 10 human fetuses (CRL, 195-300 mm; approximately 25-35 weeks of gestation). All of the fetuses studied were part of the large collection maintained at the Embryology Institute of the Universidad Complutense, Madrid, and are the products of urgent abortions, miscarriages, or ectopic pregnancies managed at the Department of Obstetrics of the University. Approval for the study was granted by the University ethics committee. The donated fetuses were fixed in 10% v/v formalin solution for more than 3 months. After decalcification with Plank-Rychlo solution (AlCl2/6H2O, 7.0 w/v%; HCl, 3.6; HCOOH, 4.6; Wako, Tokyo, Japan) for 2-5 days at room temperature, the head was divided along the midsagittal line, and one half was used for study. The upper part of the head above the eye, as well as the face, was removed in order to reduce the size of the section. A routine procedure for paraffin-embedded histology was performed. Two adjacent sections (each 7 µm thick) were obtained, at intervals of 100-200 mm: one was stained with hematoxylin and eosin (H&E), and the other was stored for immunohistochemical analysis. S100 protein immunohistochemical analysis was performed on 5-30 sections per specimen, selected on the basis of observations of the H&E stained preparations. The primary antibody used was mouse monoclonal anti-human S100 protein (1:100, Dako Z0311, Dako, Glostrup, Denmark). The secondary antibody was labeled with horseradish peroxidase (HRP), and antigen-antibody reactions were detected by the HRP-catalyzed reaction with diaminobenzidine. All samples were counterstained with hematoxylin. Negative controls consisted of samples treated as described above, but excluding the primary antibody.
In all of the 10 specimens, immunohistochemical analysis for S100 protein revealed thin nerves not only in the pharyngeal walls, but also in the surrounding structures, such as the palate, tongue, larynx, middle ear, jugular foramen, and prevertebral muscles. However, non-specific staining of muscles was generally strong. From the specimens, the clearest images with little background (obtained from 1 specimen) are shown in Figs. 2-4; thus, the presentation begins from the peripheral or medial side of the nerve courses.
Pharyngeal nerves were identified as the numerous dots in the CPS and CPM (Figs. 2, 3). Multiple pharyngeal nerves (3-5 in number) in the CPS entered the LVP at the most peripheral site of the nerve courses (Fig. 2A). Because the CPS and LVP remained attached, the nerves appeared to maintain their intramuscular courses from the CPS to the LVP. In the LVP, the nerves displayed a unique circular course toward the posterior side, and extended laterally within the muscle (Fig. 4). Likewise, the other 2-3 nerves in the CPS extended to the palatopharyngeus muscle (Fig. 2C): these 2 muscles were also still attached to the constrictors. More laterally, a cluster of dots (i.e., nerves) was observed in the CPM and the inferior part of the CPS; these nerves formed a plexus-like structure within these muscles (Figs. 2E, 3A). Part of this plexus appeared to give off twigs to the constrictor pharyngis inferior muscle, but the upper border of the muscle was not clearly defined (Fig. 2E, F). Notably, during their courses from the CPM to the LVP, the nerves did not run along the posterior surface of the pharyngeal muscles, but rather ran intramuscularly. Most of the pharyngeal nerves appeared to enter the CPM initially, and then extend to the CPS secondarily, while a few entered the CPS directly (Fig. 3C, E). The nerves described above are summarized in Fig. 5.
In areas lateral to the greater horn of the hyoid bone (Fig. 3C, E), the pharyngeal nerves joined together to form several thick nerve bundles posterior to the CPM. Behind the greater horn, the pharyngeal nerves joined the lingual branches of the glossopharyngeal nerve. Although this confluence is not shown in the figures (lateral to Fig. 3E), it was located immediately superior to the nodose ganglion of the vagus nerve [12, 13]. The pharyngeal nerves would be expected to communicate with the vagus nerve branches, but because we used semi-serial sections cut at 100- to 200-µm intervals, we were unable to investigate the actual morphology of this communication. Therefore, Fig. 5 does not include the vagus nerve. Nerves passing through the palatine canals did not reach the CPS and LVP. The tensor veli palatini muscle was located outside of (superior to) the areas shown in Figs. 2 and 3. In contrast to the narrow pharyngeal cavity, the pharyngeal recess, also known as Rosenmüller's fossa, was relatively large (Fig. 2B, D), and contained abundant glands (Fig. 3B). Unlike the situation in adults, the jugular foramen was evident behind the hyoid bone (Fig. 3F), while the tongue was located in front of the pharyngotympanic tube (Fig. 3B). We were not able to identify a nerve passing through the stylopharyngeus muscle to supply the CPS [6].
Overall, the pharyngeal nerves, as well as the lingual branches of the glossopharyngeal nerve, exhibited a curved or recurrent course; they originated from the nerve trunks at the supero-inferior level including the hyoid bone, turned upward, and reached the superior level, including the pharyngotympanic tube. The nerves were located in the deeper side of the CPS and CPM in the more medial sections. Although Fig. 5 does not depict the intramuscular courses clearly, we should emphasize that the nerve courses through the CPM-CPS-LVP, and from the CPS to the palatopharyngeus muscle were embedded within those muscles, and did not run along their external surfaces.
Although Doménech-Ratto [10] used the term "plexus pharyngeus" in a study of smaller fetuses, the pharyngeal nerves in the larger fetuses we studied here were arranged much more densely in the pharyngeal wall (and not outside of it). The nerve density was highest in the CPM. No such cluster of nerves, or plexus, is seen in the fetal tongue at the same stage [11]. We confirmed the curved or recurrent courses of the pharyngeal nerves that had been previously described by Shimokawa et al. [6]. Likewise, we confirmed that there was a common nerve supply to the CPS and LVP. However, in contrast to previous descriptions, the pharyngeal nerves, extending from the CPM via the CPS to reach the LVP, took an intramuscular course once they had entered the CPM at an inferior level near the hyoid bone. Conversely, no, or only a few nerves seemed to run along the posterior surface of these muscles. Thus, the currently described pharyngeal nerves appeared to be proper muscular nerves and were considered unlikely to contain mucosal nerves because of the wide separation between their intramuscular course and the mucosa. Therefore, it appeared that the so-called pharyngeal plexus was not part of the enteric plexus, but simply an intramuscular nerve network. The pharyngeal nerve plexus in adults may be established by exposure of the fetal intramuscular nerves to the posterior aspect of the pharyngeal wall because of muscle degeneration and subsequent rearrangement of the topographical relationship between the muscles after birth. We speculate that the suggested rearrangement is similar to a positional change from "piling tiles" to "roofing tiles." Although the lingual branches of the glossopharyngeal nerve exhibit an almost straight course in adults (Fig. 1), they took curved courses in the fetuses studied here (Fig. 5). Thus, the topographical relationship between the pharyngeal walls and the tongue might also undergo a drastic change after birth.
Shimokawa et al. [6] classified the morphology of the pharyngeal nerve into three types according to origin (the glossopharyngeal nerve, vagus nerve, or both). However, because of technical limitations, we were unable to identify any communication between the glossopharyngeal and vagus nerves behind the hyoid bone. Several Japanese research groups [6, 14, 15] have reported that the pharyngeal nerves running to the CPS are arranged lateromedially in adults. However, in this series of fetuses, the lateromedial direction did not correspond to the "extramuscular" nerve arrangement, but rather to the intramuscular course from the CPM-CPS-LVP. Therefore, a significant change in muscle topography after birth again seems likely (see the paragraph above). Additionally, a number of investigators have suggested that the facial nerve branch supplies the LVP, especially via the minor palatal nerves [16-19]. However, the present immunohistochemical study did not reveal any nerve connecting the LVP to the palatal region. In addition, lateral sections have been provided in our recent publications [12, 13].
Figures and Tables
Fig. 1
Previous descriptions of the pharyngeal nerve plexus in adults: two conflicting viewpoints. Posterior view. Panel (A) is a modification of Fig. 1319 from the atlas by Rosa and Toldt [5], while panel (B) shows our understanding of the description given by Shimokawa et al. [6]. In panel (A), most of the nerve branches supplying the pharyngeal muscles exhibit downward courses along the posterior surface, except for an ascending branch (indicated by arrows) of the glossopharyngeal nerve (IX). In panel (B), most of the nerves to the pharyngeal muscles display curved courses and terminal twigs, including those to the levator veli palatini muscle (LVP), and run upward along the posterior surface. C1, C2, C4, C5, the first, second, fourth, or fifth cervical nerve root; CPI, constrictor pharyngis inferior muscle; CPM, constrictor pharyngis medius muscle; CPS, constrictor pharyngis superior muscle; LB-IX, lingual branches of the glossopharyngeal nerve; SCG, superior cervical ganglion of the sympathetic trunk; SP, stylopharyngeus muscle; X, vagus nerve; XII, hypoglossal nerve.
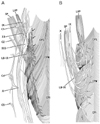
Fig. 2
Nerve distribution in the upper pharyngeal muscles: medial parts. Sagittal sections from a fetus at 30 weeks. S100 protein immunohistochemical analysis (A, C, E) and hematoxylin and eosin staining of the adjacent section (B, D, F). Panels (A) and (B) display the most medial side of Figs. 2 and 3, while panels (E) and (F) show the most lateral plane in this figure. Panels (A), (C), and (E) (panels B, D, and F) are prepared at the same magnification (see scale bar in A or B). Intervals between the panels are 0.3 mm (A-C) and 0.5 mm (C-E). Squares including the levator veli palatini muscle (LVP) are shown in Fig. 4 at a higher magnification. Some pharyngeal nerves (arrows in A) in the constrictor pharyngis superior muscle (CPS) enter the LVP. In panel (C), some nerves in the CPS (arrowheads) appear to extend to the palatopharyngeus muscle (PP). In panel (E), the nerves form a plexus (encircled by a dotted line) in the CPS and the constrictor pharyngis medius muscle (CPM). The star indicates an aberrant muscle bundle from the CPM. The tensor veli palatini muscle is located outside of (superior to) the figure. AC, arytenoid cartilage; CC, cricoid cartilage; CPI, constrictor pharyngis inferior muscle; EP, epiglottic cartilage; LB-IX, lingual branches of the glossopharyngeal nerve; LC, longus capitis muscle; Occ, occipital bone; PCA, posterior cricoarytenoid muscle; PR, pharyngeal recess or Rosenmüller's fossa; PT, palatine tonsil; PTT, pharyngotympanic tube and its associated cartilage; PX, pharyngeal cavity; SP, stylopharyngeus muscle; RCA, rectus capitis anterior muscle; SLN, superior laryngeal nerve of the vagus nerve; VA, vertebral artery. Scale bars in (A)=2 mm (A, C, E), in (B)=4 mm (B, D, F).
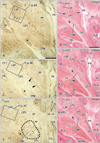
Fig. 3
Nerve distribution in the upper pharyngeal muscles: lateral parts. Sagittal sections from the same specimen shown in Fig. 2 (30 weeks). S100 protein immunohistochemical analysis (A, C, E) and hematoxylin and eosin staining of the adjacent section (B, D, F). Panels (E) and (F) display the most lateral side of Figs. 2 and 3, while (A) and (B) show the most medial plane in this figure. Panels (A), (C), and (E) (or B, D, and F) are prepared at the same magnification (see scale bar in A or B). Panel (A) is located 0.3 mm lateral to Fig. 2E. Intervals between the panels are 0.3 mm (A-C) and 0.7 mm (C-E). Squares including the levator veli palatini muscle (LVP) are shown in Fig. 4 at a higher magnification. In (A), most of the pharyngeal nerves (arrows and arrowheads) are still located in the constrictor pharyngis superior and medius muscles (CPS and CPM). However, in (C) and (E), relatively thick nerves (encircled by a dotted line) are concentrated on the posterior side of the CPM (C), or the greater horn of the hyoid bone (GH in panel E). In the plane 1.5 mm lateral to panel (E), the lingual branches of the glossopharyngeal nerve (LB-IX) join the pharyngeal nerves behind the greater horn. The star indicates an aberrant muscle bundle from the CPM. The asterisk in (C) indicates tissue damage that occurred during the histological preparation. AC, arytenoid cartilage; CC, cricoid cartilage; CPI, constrictor pharyngis inferior muscle; CPM, constrictor pharyngis medius muscle; CPS, constrictor pharyngis superior muscle; HB, hyoid body; HG, hyoglossus muscle; JF, jugular foramen (invisible); LC, longus capitis muscle; LH, lesser horn of the hyoid bone; Occ, occipital bone; PCA, posterior cricoarytenoid muscle; PP, palatopharyngeus muscle; PT, palatine tonsil; PTT, pharyngotympanic tube and its associated cartilage; PX, pharyngeal cavity; RCA, rectus capitis anterior muscle; SG, styloglossus muscle; SP, stylopharyngeus muscle. Scale bars in (A)=2 mm (A, C, E), in (B)=4 mm (B, D, F).
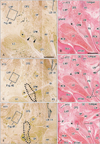
Fig. 4
(A-F) Nerve distribution in the levator veli palatini muscle. Sagittal sections from the same specimen shown in Figs. 2 and 3 (30 weeks). S100 protein immunohistochemistry. Each panel corresponds to the square including the levator veli palatini muscle (LVP) in Figs. 2 and 3. Panel (A) (panel F) is the most medial (lateral) in the figure. Multiple nerves in the constrictor pharyngis superior muscle (CPS) enter the LVP at the medial site, corresponding to Fig. 2A. These nerves follow a circular course toward the posterior side and extend laterally in the muscle. Scale bar in (A)=1 mm (A-F).
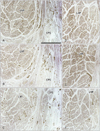
Fig. 5
Hypothetical diagram showing the glossopharyngeal nerve supply to the pharyngeal muscles. Lateral view. A nerve branch gives off twigs to the constrictor pharyngis medius and superior muscle (CPM and CPS) and reaches the levator veli palatini muscle (LVP), while another supplies the constrictor pharyngis inferior (CPI), the palatopharyngeus muscle (PP), and the CPM, before finally reaching the CPS. For clarity, we have shown the nerve branches on the outside of the muscles, but note that they did not actually run along the surfaces of the CPM, CPS, LVP, and PP, but rather took intramuscular courses. Likewise, there was no nerve plexus in the posterior aspect of the muscles. LB-IX, lingual branches of the glossopharyngeal nerve (IX); SG, styloglossus muscle; SP, stylopharyngeus muscle.
Acknowledgements
This research was supported by an Oral Health Science Center Grant hrc8 from Tokyo Dental College, and by a Project for Private Universities matching fund subsidy from Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan, 2010-2012.
References
1. Williams PL. Gray's anatomy. 1995. 38th ed. Edinburgh: Churchill Livingstone.
2. Moore KL, Dalley AF, Agur AM. Clinically oriented anatomy. 1999. London: Lippincott Williams & Wilkins;959–961.
3. Ohtsuka K, Tomita H, Murakami G. Anatomy of the tonsillar bed: topographical relationship between the palatine tonsil and the lingual branch of the glossopharyngeal nerve. Acta Otolaryngol Suppl. 2002. (546):99–109.
4. Oda K, Takanashi Y, Katori Y, Fujimiya M, Murakami G, Kawase T. A ganglion cell cluster along the glossopharyngeal nerve near the human palatine tonsil. Acta Otolaryngol. 2013. 133:509–512.
5. Rosa AD, Toldt C. Anatomischer atlas: fur Studierende und Ärzte. 1903. Vol. 6. Berlin and Wien: Urban & Schwarzenberg.
6. Shimokawa T, Yi SQ, Izumi A, Ru F, Akita K, Sato T, Tanaka S. An anatomical study of the levator veli palatini and superior constrictor with special reference to their nerve supply. Surg Radiol Anat. 2004. 26:100–105.
7. Hamilton WJ, Mossman HW. Hamilton, Boyd and Mossmam's human embryology: prenatal development of form and function. 1978. 4th ed. London: Williams & Wilkins;291–335.
8. Skandalakis JE, Gray SW. Embryology for surgeons: the embryological basis for the treatment of congenital anomlaies. 1994. 2nd ed. Baltimore: Williams & Wilkins;17–64.
9. O'Rahilly R, Müller F. Human embryology and teratology. 1996. 2nd ed. New York: Wiley-Liss;317–320.
10. Doménech-Ratto G. Development and peripheral innervation of the palatal muscles. Acta Anat (Basel). 1977. 97:4–14.
11. Abe S, Kikuchi R, Nakao T, Cho BH, Murakami G, Ide Y. Nerve terminal distribution in the human tongue intrinsic muscles: an immunohistochemical study using midterm fetuses. Clin Anat. 2012. 25:189–197.
12. Katori Y, Kawase T, Ho Cho K, Abe H, Rodríguez-Vázquez JF, Murakami G, Fujimiya M. Suprahyoid neck fascial configuration, especially in the posterior compartment of the parapharyngeal space: a histological study using late-stage human fetuses. Clin Anat. 2013. 26:204–212.
13. Katori Y, Kawase T, Cho KH, Abe H, Rodríguez-Vázquez JF, Murakami G, Abe S. Prestyloid compartment of the parapharyngeal space: a histological study using late-stage human fetuses. Surg Radiol Anat. 2012. 34:909–920.
14. Fuse S. An anatomical study of glossopharyngeal nerve. Acta Anat Niigata. 1952. 29:148–188.
15. Murakami K, Kuroda M, Kishi K. Variations of the constrictor pharyngeal muscles in humans. Kaibogaku Zasshi. 1996. 71:638–649.
16. Futamura R. Über die Entwicklung der facialis muskulatur des Menschen. Anat Hefte. 1906. 30:436–516.
17. Podvinec S. The physiology and pathology of the soft palate. J Laryngol Otol. 1952. 66:452–461.
18. Ibuki K, Matsuya T, Nishio J, Hamamura Y, Miyazaki T. The course of facial nerve innervation for the levator veli palatini muscle. Cleft Palate J. 1978. 15:209–214.
19. Leonhaldt H, Tillman B, Töndury G, Zilles K. Rauber-Kopsch Anatomie des Menschen, Lehrbuch und Atlas, Bd. II Innere Organe. 1987. Stuttgart: Georg Thieme Verlag.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download