Abstract
In recent years, examination and comparison of the biological characteristics of bone marrow- and adipose-derived mesenchymal stem cells (MSCs) from various perspectives have come into the focus of stem cell research, as these cells should be well characterized in order to utilize them in future cellular therapies. Therefore, in the present study, surface protein markers and the skeletal myogenic differentiation potential of rat bone marrow- and adipose-derived MSCs were examined. The expression of CD44, CD45, CD73, and CD90 on bone marrow- and adipose-derived MSCs was characterized using flow cytometry. Subsequently, the stem cells were differentiated into myogenic lineages, and the expression of the skeletal myogenic markers MyoD1, Myog, and Myh2 was studied in cells using real time polymerase chain reaction and immunofluorescence. Our results reveal that the pattern of CD marker expression differs between these 2 types of MSCs to some extent, whereas no significant difference was observed with respect to their myogenic differentiation potential. Therefore, we concluded that despite the differences observed in the biological features of these 2 types of MSCs, their myogenic potential appears to be similar, and that adipose-derived stem cells may be useful in skeletal muscle tissue engineering, due to their easy isolation and capacity for rapid expansion in a short time span.
Cell-based therapy has grown rapidly over the last decade, with respect to preclinical research and in clinical trials. Embryonic and non-embryonic stem cells have been considered as potential therapeutic approaches for several types of diseases. One type of adult stem cell, the mesenchymal stem cell (MSC), has attracted a great deal of attention
in the field of regenerative medicine due to its unique biological properties [1]. Bone marrow-derived MSCs (BMSCs) were first identified in 1968 by Friedenstein as an adherent fibroblast-like cell in the bone marrow that was capable of differentiating into bone [2]. Subsequently, it was shown that MSCs can be isolated from various tissue sources such as adipose tissue, peripheral blood, umbilical cord, and placenta. These cells possess a considerable capacity for in vitro expansion, which allows them to rapidly reach numbers sufficient for cell-based therapy [3].
Adipose tissue is highly complex and contains mature adipocytes, preadipocytes, fibroblasts, vascular smooth muscle cells, endothelial cells, resident monocytes/macrophages, and lymphocytes. The stromal-vascular cell fraction of adipose tissue has increasingly come into focus in stem cell research, since this compartment represents a rich source of multipotent adipose-derived stem cells (ASCs) [4]. Because ASCs are of mesodermal origin, they can differentiate into adipogenic, osteogenic, chondrogenic, and myogenic lineages, which can yield skeletal and smooth muscle cells as well as cardiomyocytes. Interestingly, ASCs have also been shown to possess the potential to differentiate into nonmesodermal lineages, including neuron-like cells, endothelial cells, epithelial cells, hepatocytes, pancreatic cells, and hematopoietic supporting cells [5, 6]. To identify the best source of MSCs for future application in regenerative medicine, BMSCs and ASCs have been recently studied and compared from multiple different research perspectives. They have been examined in a myocardial infarction model [7], expanded on a nanoparticle-coated substratum [8], and subjected to chondrogenic differentiation [9]. Additionally, the expression of cell surface markers [10], sensitivity to chemotherapeutic agents [11], morphological, molecular and functional differences [12], biological characteristics and multilineage differentiation [13], the impact of cell source, culture methodology, culture location, and individual donors on gene expression profiles [14], and the effects of cyclic hydrostatic pressure on chondrogenesis and cell viability [15] have also been studied. MSCs with myogenic potential that can fuse with co-cultured myoblasts to produce myotubes in vitro can be obtained from 2 sources, bone marrow and adipose tissues. These cells also showed myogenic regenerative potential when transplanted in vivo, even in the mdx mouse model of Duchenne muscular dystrophy [16, 17]. However, up to now, few studies have been conducted to compare the myogenic differentiation potentials of BMSCs and ASCs. Moreover, the selection of suitable sources of MSCs is vital for future in vitro and in vivo experiments in the field of skeletal muscle tissue engineering. Therefore, we isolated rat MSCs from bone marrow and adipose tissues to compare their ease of isolation, the expression of surface protein markers, and more importantly, their differentiation potential into skeletal myogenic lineages, using markers that have not been used in previous studies.
All animal experiments were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. For BMSC isolation, hind limbs of Albino rats (8-week-old males) were dissected and maintained in ice-cold Hanks balanced salt solution (HBSS; Sigma-Aldrich, St. Louis, MO, USA) under sterile conditions. After removal of the musculature and connective tissue, the femur and tibia were rinsed 3 times with HBSS, and their epiphyses were carefully cut with a bone cutter. The bone marrow was flushed using a 10 ml syringe containing alpha-minimum essential media (α-MEM) (Gibco, Grand Island, NY, USA). The mononucleated cell layer of bone marrow containing MSCs was separated using Ficoll-Paque (Pharmacia Fine Chemicals, Piscataway, NJ, USA) and centrifugation for 20 minutes at 2,500 rpm at room temperature. Mononucleated cells isolated at this step were re-centrifuged at 1,600 rpm for 5 minutes; thereafter, the supernatant was removed and the pellet was suspended in α-MEM supplemented with 10% fetal bovine serum (FBS; Gibco), penicillin/streptomycin (Sigma-Aldrich), and L-glutamine (1%; Gibco) and incubated (37℃, 5% CO2) until confluent. After the initial 24 hours, fresh medium was added to the cells every 72 hours. At confluence, the cells were detached using 0.25% trypsin (Sigma-Aldrich) containing 0.1% ethylenediaminetetraacetic acid (Sigma-Aldrich) and subcultured at a density of 4,000 cells/cm2.
To isolate ASCs, gonadal fat pads of Albino rats (8-week-old males) were dissected. Adipose tissue was washed extensively with phosphate-buffered saline (PBS; Gibco) and minced with fine scissors. The tissue was digested with 0.1% collagenase type I solution (Invitrogen, Carlsbad, CA, USA) at 37℃. After partial digestion, the collagenase activity was neutralized by adding α-MEM (Gibco) supplemented with 10% FBS, and the resulting cell suspension was filtered through a 100-mm cell strainer and centrifuged at 2,000 rpm for 5 minutes. Cell pellets were resuspended in α-MEM supplemented with 10% FBS, penicillin/streptomycin, and L-glutamine (1%) and incubated at 37℃, 5% CO2. After 24 hours, unattached cells and debris were removed by aspiration, and fresh medium was added to the adherent cells. Thereafter, the medium was changed twice a week until the cells reached 80% confluence. At confluence, the cells were subcultured as above.
After 4 passages, the potency and the surface markers of isolated BMSCs and ASCs were assessed using multilineage differentiation assays and flow cytometry, respectively.
Differentiation assays were performed to determine the multipotentiality of the BMSCs and ASCs. Adipogenic differentiation of cells was determined using oil red O staining (Sigma-Aldrich) after culturing cells in an adipogenic medium containing 1 µM dexamethasone (Sigma-Aldrich), 500 µM isobutylmethylxanthine (Sigma-Aldrich), 60 µM indomethacin (Sigma-Aldrich), and 5 µg/mL insulin (Sigma-Aldrich) for 3 weeks.
Mineralized colonies resulting from the osteogenic differentiation of cells were visualized with alizarin red S staining (Sigma-Aldrich), after culturing the cells in an osteogenic differentiation medium containing 50 µM ascorbate-2 phosphate (Sigma-Aldrich), 10 mM β-glycerophosphate (Sigma-Aldrich), and 0.1 µM dexamethasone for 3 weeks.
The expression of surface antigens CD44, CD73, and CD90 (as positive markers) and CD45 (as a negative marker) on cells was evaluated by flow cytometry using the following antibodies: FITC-conjugated mouse anti-rat CD44 (BD Bioscience, San Jose, CA, USA), FITC-conjugated mouse anti-rat CD45 (eBioscience, San Diego, CA, USA), fluorescein isothiocyanate-conjugated mouse anti-rat CD90 (eBioscience), FITC-conjugated mouse IgG2a isotype control (eBioscience), FITC-conjugated mouse IgG1 isotype control (eBio-science), purified mouse anti-rat CD73 (BD Bioscience), affinity-purified mouse IgG1 isotype control (eBioscience), and PE-conjugated donkey anti-mouse IgG (H+L) (eBioscience). Unconjugated antibodies were incubated with the secondary phycoerythrin-conjugated antibody for an additional 15 minutes. Gating was set using unstained cells, and in each analysis, at least 15,000 events were collected. Finally, the obtained data was analyzed using FlowJo software version 7.6.4 (Tree Star, Ashland, OR, USA).
There were 2 groups in total for each type of MSC in the study, and the experiments were performed within 3 days. In the myogenic differentiation group, differentiation was induced chemically by growth factors (5-azacytine and horse serum), while in the control group, cells were cultured in the proliferation medium without any added growth factors.
Immediately after completion of the experiment, the cells cultured on coverslips were rinsed with ice cold PBS, fixed using 4% paraformaldehyde (pH 8.0, Sigma-Aldrich) for 20 minutes, and permeabilized with 0.5% Triton X 100 (Amersham, Piscataway, NJ, USA) for 10 minutes. Next, cells were blocked in 10% normal goat serum (Sigma-Aldrich) and incubated with mouse monoclonal anti-MyoD1 (1:100), mouse monoclonal anti-myogenin (1:100), or mouse monoclonal anti-myosin (fast skeletal, 1:100, Sigma-Aldrich) overnight at 4℃. Thereafter, cells were rinsed with PBS 3 times and incubated with FITC-conjugated goat anti-mouse antibody (1:100, Sigma-Aldrich) for 2 hours at room temperature in the dark. Finally, the coverslips were mounted on the glass slide and examined under a Zeiss fluorescence microscope (×630). Differentiation was evaluated qualitatively, through an inspection of immunofluorescent images, using Adobe Photoshop version 8.0.
Total RNA was extracted from cells using the RNeasy Plus Mini Kit (Qiagen, Gathersburg, MD, USA), quantified using a PicoDrop spectrophotometer (Pico100, Picodrop, Saffron Walden, UK), and then stored in RNase-free water at -80℃. The cDNA was synthesized using a QuantiTect Reverse Transcription Kit (Qiagen) according to manufacturer's instructions. SYBER green-based RT-PCR primers were designed to span exon/intron junctions using Primer Express Software (version 2.5). The sequences of primers were as follows: MyHC2, forward 5'-ggctggctggacaagaaca-3' and reverse 5'-ccaccactacttgcctctgc-3'; MyoD1, forward 5'-tggcatgatggattacagcg-3' and reverse 5'-actcttccctggtctgggc-3'; Myog, forward 5'-cggtggtacccagtgaatgc-3' and reverse 5'-gctgcgagcaaatgatctcc-3'; β-actin, forward 5'-agccatgtacgtagccatcca-3' and reverse 5'-tctccggagtccatcacaatg-3'. SYBR Premix Ex Taq (Takara Bio Inc., Otsu, Shiga, Japan) was used according to its protocol with a LightCycler for two-step RT-PCR. The obtained data was analyzed using the comparative threshold cycle (CT), in which the formula 2-ΔΔCT was used as ΔCT=CT of target gene-CT of housekeeping gene (normalization) and ΔΔCT=ΔCT of sample-ΔCT of the calibrator (control).
Statistical analysis was performed using SPSS version 16 (SPSS Inc., Chicago, IL, USA). All data (obtained from 3 independent experiments performed in triplicate) were presented as mean±SE. One-way ANOVA and Duncan's post-hoc test were used to compare the groups. P<0.05 was considered to be statistically significant.
Two types of cells were observed in culture flasks 24 hours after isolation of the BMSCs. A small number of suspended cells (which appeared to be red blood cells) were seen, while the majority of cells were adherent and appeared round, although a few cells showed processes (Fig. 1A, B). After 5 days, the adherent cells became active, formed processes, and expanded to generate small and large colonies with a heterogeneous appearance. These colonies seemed to be formed from single fibroblast-like cells, the so-called colony-forming unit fibroblasts. On examining the adherent cell morphology with inverted microscopy, 2 morphologies were identifiable. Some cells were small and appeared round, fusiform, or trigonal, while others were large cells with a multiform and polygonal cytoplasm (Fig. 2A, B). Two weeks later, the cells reached 80% confluence and were passaged. Heterogeneity was the most significant feature of the cells in the early passages. After the forth passage, cells were mostly homogenous in shape and size.
On the first day of ASC isolation, a variety of cell populations with different sizes and morphologies appeared in the culture; there seemed to be more of these populations than after the first day of BMSC isolation. Cells reached confluence in less than a week and were subsequently passaged. After the fourth passage, cells became homogenous in shape and size (Fig. 3A, B).
Based on the adherent properties of BMSCs and ASCs, these cells were extracted from the hind limb bone marrow and the gonadal fat pads, respectively, and characterized by flow cytometry by examining the expression of common stem cell surface markers. After the fourth passage, the majority of BMSCs expressed the mesenchymal cell surface markers CD90 and CD44; however, the mesenchymal cell surface marker CD73 was not expressed in a fraction of cells. In contrast, almost all ASCs expressed CD73 and CD90, but only a fraction of ASCs expressed CD44 on the cell surface. The majority of BMSCs and ASCs were negative for CD45, a hematopoietic cell surface marker (Fig. 4).
Myogenic differentiation analysis by immunofluorescence demonstrated that neither BMSCs nor ASCs in the control groups were positive for MyoD1, Myog, or fast skeletal myosin. Additionally, there was no substantial qualitative difference between myogenically differentiated BMSCs and ASCs when they were examined by fluorescence microscopy, or when their images were further examined using Adobe Photoshop software (Fig. 5A-C). The expression levels of MyoD1, Myog, and Myh2 mRNA in BMSCs and ASCs were examined by RT-PCR (Fig. 6). The expression of Myog and Myh2 did not differ significantly between the BMSCs and ASCs. However, MyoD1 expression tended to be significantly higher in the BMSCs than in the ASCs (P<0.05).
The MSC has been an extremely attractive cell model for research into the treatment of a variety of diseases over the past decade. Currently, MSC-based clinical trials have been performed for at least 12 types of pathological conditions, and many of these trials have demonstrated the safety and efficacy of MSCs. The biological characteristics of MSCs that contribute to their therapeutic effects need to be identified in order for them to be used in clinical applications. Currently, the following 4 features of MSCs are considered to be the most important: 1) the ability to reside in injured tissues when injected intravenously, 2) the ability to differentiate into various cell types, 3) the ability to secrete a wide variety of bioactive molecules that act in the repair of injured cells and inhibit inflammation, and 4) the lack of immunogenicity and an immunomodulatory capacity [2, 3]. Finding the best source of MSCs for future use in stem cell-based therapies has been the subject of many recent studies. These studies have isolated BMSCs and ASCs from various species and compared them with respect to various aspects [7-15]. However, there are 2 main issues remaining, which our work has addressed: first, few studies have been conducted on the biological characteristics of rat BMSCs and ASCs, and second, few studies have been performed to compare the myogenic potential of BMSCs and ASCs from human and nonhuman species. Our data demonstrated 2 main characteristics of rat BMSCs and ASCs: first, there were substantial differences in their expression patterns of surface proteins; and second, no substantial differences were evident in their myogenic differentiation potential.
Based on standards proposed by the Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy (ISCT), 3 main criteria have been defined to characterize MSCs: 1) adherence to plastic cultureware when cultured under standard conditions; 2) expression of surface proteins such as CD90, CD73, and CD105 and a lack of expression of CD45, CD34, CD14 or CD11b, CD79a or CD19, and HLA class II; and 3) an ability to differentiate into osteoblasts, adipocytes, and chondroblasts when cultured in specific differentiating media [18]. The committee emphasized that these criteria exclusively apply to human MSCs and that adherence and trilineage differentiation are not necessary for characterization of MSCs from other species. However, in the present study, all these criteria were considered for the characterization of isolated BMSCs and ASCs. Both types of stem cells displayed good adherence after 1 day in culture and also differentiated in due time into osteoblasts, adipocytes, and chondroblasts when cultured in specific osteogenic, adipogenic, and chondrogenic culture media. In previous studies in rats, the expression of CD44, CD73, CD90, and CD45 on isolated BMSCs and ASCs was studied. The results indicated that all BMSCs were positive for CD44 and CD90, whereas most, but not all, cells expressed CD73 on their surface; the pattern reported for CD73 expression is consistent with our results [19, 20]. All ASCs were positive for CD73 and CD90, whereas CD44 was only expressed in a fraction of ASCs. Previous studies on rat ASCs support our findings on CD44 expression [21]. However, it is noteworthy that MSCs from other species do not express all the same markers as found on human cells; for example, although human and rat MSCs have been shown to be CD34, some papers report variable expression of this marker on murine MSCs [22]. It is generally accepted that MSCs do not express the hematopoietic marker CD45 and the endothelial cell marker CD31 [3]. However, it is important to note that differences in the cell surface expression of many markers may be influenced by factors secreted by accessory cells in the initial passages, variation in tissue source, the method of isolation and culture, and species-related differences [23-25]. For example, CD49d is expressed on human ASCs but not BMSCs, and CD106 is expressed on BMSCs but not ASCs. It has been shown in previous studies that CD106 expression on MSCs in bone marrow is functionally linked with hematopoiesis, so the lack of CD106 expression on ASCs is associated with the localization of these cells in nonhematopoietic tissues [3, 26]. It was reported that CD44, which is expressed on MSCs, is an important receptor that is involved in MSC migration through interaction with hyaluronan. Zhu et al. [13] have studied the role of CD44-hyaluronan interactions for BMSC migration. They found that CD44 expression was elevated on BMSCs upon platelet-derived growth factor stimulation and their adhesion and migration on hyaluronan was undoubtedly dependent on CD44, as it could be blocked by either an antibody to CD44 or a small interfering RNA [27].
Our data revealed that both BMSCs and ASCs differentiated into myogenic lineages, and no significant difference was observed between them in this regard. These results apparently conflict with a previous study by Meligy et al. [28] in which the myogenic differentiation of rat MSCs derived from bone marrow, adipose tissue, and skeletal muscle was compared. After a week of induction, myogenin expression was highest in skeletal muscle MSCs. The expression of myogenin was also higher in BMSCs than ASCs, but ASCs were reported to be more accessible than the other 2 types of MSC and have the highest rate of growth in culture. The results of that study also indicated that stem cell marker expression was higher in ASCs than BMSCs. This is obviously different from our results. However, 2 main differences exist between our work and this study. First, Meligy et al. [28] studied Sprague-Dawley rats whereas we used Wistar rats. Second, we used a different method for myogenic induction, i.e., 24 hours with 5-azacytidine followed by 2% horse serum. Recently, it was shown that human MSCs from bone marrow, adipose tissue, and synovial membranes exhibited similar myogenic properties in vitro but, when transplanted into cardiotoxin-damaged tibialis anterior muscles of immunodeficient mice, ASCs generated a high frequency of hybrid myofibers [29].
Despite previously described biological similarities between BMSCs and ASCs, many recent studies have compared different aspects of these cells, which is indicative of the importance of these cells for promising applications in tissue engineering research and the need to choose the best option for future cell-based therapies. Recent studies revealed several similarities and differences between BMSCs and ASCs. For example, ASCs are able preserve cardiac function following myocardial infarction but the BMSCs are not [7]; both BMSCs and ASCs adhered to and proliferated similarly on nanoparticle-coated plates [8]; both stem cells presented a highly similar morphology and marker expression in an undifferentiated state [9, 10], but compared to ASCs, BMSCs showed an enhanced capacity to differentiate into the chondrogenic lineage [9]; ASCs displayed more resistance to chemotherapy than BMSCs and might therefore be more advantageous for application in clinical settings [11]; cell origin and abundance were determined as vital factors in stem cell selection, and it was found that adipose tissue is a more promising source of stem cells [4, 13, 14]; and finally, cyclic hydrostatic pressure could induce human ASCs to undergo chondrogenic differentiation in a manner similar to BMSCs [15]. Taken together, past and present studies indicate that, despite some differences between BMSCs and ASCs in their expression of surface proteins markers, it seems that their myogenic potential is similar (albeit not identical); and it also suggests that ASCs may be a good candidate for use in skeletal muscle tissue engineering research because they could be isolated in abundant quantities from a tissue which can be obtained by a procedure that is minimally invasive, in comparison to that required for BMSCs.
Figures and Tables
 | Fig. 1Mononuclear cells isolated from bone marrow after 24 hours in culture. (A) The majority of adherent cells were round (arrowheads) (inverted microscope, ×100) although (B) occasionally some of them had processes (arrows) (inverted microscope, ×250). |
 | Fig. 2Bone marrow-derived mesenchymal stem cells (BMSCs) after 5 days in culture. (A) A colony of BMSCs on the fifth day (inverted microscope, ×100). (B) By observing with high magnification, BMSCs seemed to contain cells with 2 different morphologies: small cells with various appearances and large cells that were multiform and polygonal. Arrow points to a large cell (inverted microscope, ×250). |
 | Fig. 3Stromal-vascular cell fraction (SVF) of the adipose tissue on the first day of culture. (A) A variety of cell populations with different sizes and morphologies appeared in the culture. (B) SVF cells after reaching confluence in less than 1 week (inverted microscope, ×250). |
 | Fig. 4Flow cytometric results demonstrated that the bone marrow-derived mesenchymal stem cells (BMSCs) and adipose-derived stem cells (ASCs) differed in the expression of CD44 and CD73 on their surfaces. |
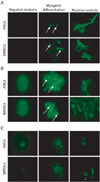 | Fig. 5Expression and the intracellular position of myogenic markers MyoD1, Myog, and myosin (fast skeletal) in bone marrow-derived mesenchymal stem cells (BMSCs) and adipose-derived stem cells (ASCs) in the absence or presence of chemical growth factors. MyoD1 (A) and Myog (B), as skeletal muscle transcription factors, were mostly concentrated in the nuclei (arrows). (C) Myosin (fast skeletal). Undifferentiated stem cells were taken as a negative control, and the cell line L6 was considered as positive control (A-C, ×630). |
References
1. Ahn SM, Simpson R, Lee B. Genomics and proteomics in stem cell research: the road ahead. Anat Cell Biol. 2010. 43:1–14.
2. Wang S, Qu X, Zhao RC. Clinical applications of mesenchymal stem cells. J Hematol Oncol. 2012. 5:19.
3. Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007. 25:2739–2749.
4. Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007. 100:1249–1260.
5. Bunnell BA, Flaat M, Gagliardi C, Patel B, Ripoll C. Adipose-derived stem cells: isolation, expansion and differentiation. Methods. 2008. 45:115–120.
6. Fraser JK, Wulur I, Alfonso Z, Hedrick MH. Fat tissue: an underappreciated source of stem cells for biotechnology. Trends Biotechnol. 2006. 24:150–154.
7. Rasmussen JG, Frobert O, Holst-Hansen C, Kastrup J, Baandrup U, Zachar V, Fink T, Simonsen U. Comparison of Human Adipose-Derived Stem Cells and Bone Marrow-Derived Stem Cells in a Myocardial Infarction Model. Cell Transplant. 2012. 12. 4. [Epub] http://dx.doi.org/10.3727/096368912X659871.
8. Kishimoto S, Ishihara M, Mori Y, Takikawa M, Hattori H, Nakamura S, Sato T. Effective expansion of human adipose-derived stromal cells and bone marrow-derived mesenchymal stem cells cultured on a fragmin/protamine nanoparticles-coated substratum with human platelet-rich plasma. J Tissue Eng Regen Med. 2012. 03. 31. [Epub] http://dx.doi.org/10.1002/term.1488.
9. Reich CM, Raabe O, Wenisch S, Bridger PS, Kramer M, Arnhold S. Isolation, culture and chondrogenic differentiation of canine adipose tissue- and bone marrow-derived mesenchymal stem cells: a comparative study. Vet Res Commun. 2012. 36:139–148.
10. Zimmerlin L, Donnenberg VS, Rubin JP, Donnenberg AD. Mesenchymal markers on human adipose stem/progenitor cells. Cytometry A. 2013. 83:134–140.
11. Qi Z, Zhang Y, Liu L, Guo X, Qin J, Cui G. Mesenchymal stem cells derived from different origins have unique sensitivities to different chemotherapeutic agents. Cell Biol Int. 2012. 06. 14. [Epub] http://dx.doi.org/10.1002/term.1488.
12. Mantovani C, Raimondo S, Haneef MS, Geuna S, Terenghi G, Shawcross SG, Wiberg M. Morphological, molecular and functional differences of adult bone marrow- and adipose-derived stem cells isolated from rats of different ages. Exp Cell Res. 2012. 318:2034–2048.
13. Zhu X, Shi W, Tai W, Liu F. The comparition of biological characteristics and multilineage differentiation of bone marrow and adipose derived Mesenchymal stem cells. Cell Tissue Res. 2012. 350:277–287.
14. Torensma R, Prins HJ, Schrama E, Verwiel ET, Martens AC, Roelofs H, Jansen BJ. The impact of cell source, culture methodology, culture location, and individual donors on gene expression profiles of bone marrow-derived and adipose-derived stromal cells. Stem Cells Dev. 2013. 22:1086–1096.
15. Puetzer J, Williams J, Gillies A, Bernacki S, Loboa EG. The effects of cyclic hydrostatic pressure on chondrogenesis and viability of human adipose- and bone marrow-derived mesenchymal stem cells in three-dimensional agarose constructs. Tissue Eng Part A. 2013. 19:299–306.
16. Di Rocco G, Iachininoto MG, Tritarelli A, Straino S, Zacheo A, Germani A, Crea F, Capogrossi MC. Myogenic potential of adipose-tissue-derived cells. J Cell Sci. 2006. 119:2945–2952.
17. Belema Bedada F, Technau A, Ebelt H, Schulze M, Braun T. Activation of myogenic differentiation pathways in adult bone marrow-derived stem cells. Mol Cell Biol. 2005. 25:9509–9519.
18. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006. 8:315–317.
19. Semedo P, Correa-Costa M, Antonio Cenedeze M, Maria Avancini Costa Malheiros D, Antonia dos Reis M, Shimizu MH, Seguro AC, Pacheco-Silva A, Saraiva Camara NO. Mesenchymal stem cells attenuate renal fibrosis through immune modulation and remodeling properties in a rat remnant kidney model. Stem Cells. 2009. 27:3063–3073.
20. Semedo P, Palasio CG, Oliveira CD, Feitoza CQ, Gonçalves GM, Cenedeze MA, Wang PM, Teixeira VP, Reis MA, Pacheco-Silva A, Câmara NO. Early modulation of inflammation by mesenchymal stem cell after acute kidney injury. Int Immunopharmacol. 2009. 9:677–682.
21. Li B, Zeng Q, Wang H, Shao S, Mao X, Zhang F, Li S, Guo Z. Adipose tissue stromal cells transplantation in rats of acute myocardial infarction. Coron Artery Dis. 2007. 18:221–227.
22. Peister A, Mellad JA, Larson BL, Hall BM, Gibson LF, Prockop DJ. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004. 103:1662–1668.
23. Javazon EH, Beggs KJ, Flake AW. Mesenchymal stem cells: paradoxes of passaging. Exp Hematol. 2004. 32:414–425.
24. Baddoo M, Hill K, Wilkinson R, Gaupp D, Hughes C, Kopen GC, Phinney DG. Characterization of mesenchymal stem cells isolated from murine bone marrow by negative selection. J Cell Biochem. 2003. 89:1235–1249.
25. Gronthos S, Simmons PJ, Graves SE, Robey PG. Integrin-mediated interactions between human bone marrow stromal precursor cells and the extracellular matrix. Bone. 2001. 28:174–181.
26. Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002. 13:4279–4295.
27. Docheva D, Haasters F, Schieker M. Mesenchymal stem cells and their cell surface receptors. Curr Rheumatol Rev. 2008. 4:155–160.
28. Meligy FY, Shigemura K, Behnsawy HM, Fujisawa M, Kawabata M, Shirakawa T. The efficiency of in vitro isolation and myogenic differentiation of MSCs derived from adipose connective tissue, bone marrow, and skeletal muscle tissue. In Vitro Cell Dev Biol Anim. 2012. 48:203–215.
29. de la Garza-Rodea AS, van der Velde-van Dijke I, Boersma H, Gonçalves MA, van Bekkum DW, de Vries AA, Knaän-Shanzer S. Myogenic properties of human mesenchymal stem cells derived from three different sources. Cell Transplant. 2012. 21:153–173.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download