Abstract
Inflammation within the central nervous system often accompanies ischemia, trauma, infection, and other neuronal injuries. The immune system is now recognized to play a major role in neuronal cell death due to microglial activation, leukocyte recruitment, and cytokine secretion. The participation of heat shock proteins (Hsps) in the immune response following in brain injury can be seen as an attempt to correct the inflammatory condition. The Hsps comprise various families on the basis of molecular size. One of the most studied is Hsp70. Hsp70 is thought to act as a molecular chaperone that is present in almost intracellular compartments, and function by refolding misfolded or aggregated proteins. Hsps have recently been studied in inflammatory conditions. Hsp70 can both induce and arrest inflammatory reactions and lead to improved neurological outcome in experimental brain injury and ischemia. In this review, we will focus on underlying inflammatory mechanisms and Hsp70 in acute neurological injury.
Heat shock proteins (Hsps) are a family of proteins involved in many chaperone functions. Their expression can be constitutive or inducible depending on the family member. Constitutively expressed members are present in all cell compartments, and appear to assist in proper folding and assembly of polypeptide of newly synthesized proteins [1, 2]. Inducible forms were originally described following heat stress [3]; however, a variety of cellular stresses will lead to their induction as part of an orchestrated stress response. They also function as a potential cytoprotective protein. Under stressful conditions such as cytotoxic injury, heat shock, oxidative stress, radiation, viral infection, and chemicals, Hsps have long been known to serve as protein chaperones in the sense that they assist in protein folding and the correct attainment of functional three-dimensional configuration, while preventing incorrect folding and protein aggregation [4-6]. They are classified according to their molecular mass, and include Hsp100, Hsp90, Hsp70, Hsp60, Hsp40, and the small Hsp families [7]. The best-studied class is Hsp70, the 70-kDa class which includes an inducible form also known as Hsp72, Hsp70i, or simply Hsp70.
Under homeostatic conditions, Hsps are located intracellularly and are bound to heat shock factors (HSFs) [3]. HSF1 is maintained in an inactive complex with Hsp90, Hsp40, and Hsp70 [8]. Following an appropriate stress such as heat stress, accumulation of unfolded proteins leads to the dissociation of Hsps from HSF, leaving Hsps free to bind target proteins. In the stressed cell, dissociated HSF is transported to the nucleus where it is phosphorylated, possibly by protein kinase C, to form activated trimers. These trimers bind to highly conserved regulatory sequences on the heat shock gene known as heat shock elements (HSEs) [9]. Once bound to HSEs, HSFs bind to the promoter site of Hsp genes, with subsequent generation and expression of more Hsps [10]. Newly generated Hsps can then bind denatured proteins and act as a molecular chaperone by contributing to repair, refolding and trafficking of damaged proteins within the cell. Hsp90 can also influence Hsp70, since Hsp90 is bound to HSF-1. When Hsp90 dissociates from HSF-1, HSF-1 leads to Hsp70 induction.
In studies of cerebral ischemia, neurodegenerative diseases, epilepsy, and trauma, Hsp70 has been shown to be neuroprotective [8]. Through its chaperone properties, it has been shown to reduce protein aggregates [11] and intracellular inclusions [12]. Overexpression of Hsp70 in neuronal cell cultures and mouse models of neurodegenerative diseases leads to protection against a variety of acute insults such as cerebral ischemia, trauma, and hemorrhage by reducing the number and size of inclusions and the accumulation of disease-causing proteins [13, 14]. A recent paper showed that Hsp70-overexpressing transgenic mice are able to degrade proteins that accumulate in Alzheimer's disease models and leads to neurological improvement [15].
In addition to their function in protein processing, Hsps appear to play a role in cytoprotection, by affecting several cell death and immune response pathways [3, 16]. For example, overexpression of Hsp70 has been shown to reduce apoptosis [11]. Although the exact mechanism remains unclear, studies have shown that Hsp70 overexpression increases expression of the anti-apoptotic protein Bcl-2 [3], but also affects caspase processing [16].
The inflammatory response within the central nervous system (CNS) is a well known feature of a variety of acute neurological injuries including stroke, trauma, and other forms of cerebral hypoxic-ischemic insults [17]. Danger signals released in the setting of cell damage leads to a complex series of biochemical and molecular events, and have been increasingly recognized as a key contributor to cell death [18]. It is characterized by the activation of microglia and infiltration of leukocytes following brain injury [19]. Further, there is evidence that brain cells not normally viewed as immunologic, including astrocytes and even neurons, can elaborate immune molecules. This inflammatory response, particularly in the acute stage, is now recognized to contribute to brain injury and thus represents a major opportunity for investigation and to explore potential treatments.
Within a few hours after the injury onset, release of factors from damaged cells or cellular debris triggers this inflammatory response. Because of the apoplectic nature of acute brain insults, the ensuing immune response is most likely innate, rather than adaptive. The innate immune response is a triggered by a variety of signals that, unlike the adaptive immune response, do not require antigen recognition. While the list of potential activating signals grows, some recognized factors have been called danger associated molecular patterns (DAMPs) [20].
Glial cells (microglia and astrocytes) are necessary to support the central nervous system. Microglias constitute 5-20% of the total glial population and act as key modulators in this innate response [21]. They are quite sensitive to changes in the environment, and display a ramified appearance while in the resting state, but when activated, undergo a series of changes culminating in an amoeboid morphology. Microglial activation is a complex series of events, and the morphological and gene expression changes associated with microglial activation vary depending on the type, severity, and duration of the stimulus [22]. Microglial activation is the initial step in the CNS inflammatory response; depending on the stimulus, this step may be followed by infiltration of circulating monocytes, neutrophils, and lymphocytes [23]. Astrocytes will proliferate and differentiate after brain injury which can be potentially destructive. They, like microglia, lead to the production of inflammatory, where the release of cytotoxic molecules may lead to neuronal cell death [9].
The precise initiating stimulus of this acute response remains obscure, although several studies have shown that ischemia does lead to Toll-like receptor (TLR) activation, a major activating mechanism of innate immunity [24]. The actual trigger is not fully known, but reports have speculated that the injured brain releases DAMPs and other substance that trigger this response. There are reports indicating factors implicating the high mobility group box-1 (HMGB-1) [25, 26], which are related to cytokines. HMGB-1 is released by neurons and cells of the immune system in the setting of cell death. DAMPs include hyaluronan, surfactant protein, uric acid, and Hsps. These substances can then bind to and stimulate microglia and other immune cells leading to the upregulation of many immune mediators by activating several pro-inflammatory transcription factors, including nuclear factor kappa B (NF-kB) [27], hypoxia inducible factor 1, interferon regulator factor 1, and signal transducer and activator of transcription factor (STAT) 3 [28].
How immune responses are regulated are complex, but the NF-kB deserves some mention as a major and prototypic pro-inflammatory transcription factor widely expressed in the CNS [29]. Normally present in the cytosol, NF-kB is a heterodimeric protein of a p65 and p50 subunit. This dimer is bound to its endogenous inhibitor IkB, which inactivates. Upon activation by a variety of stimuli, including cytokines, oxidants and other immune factors, IkB is phosphorylated by its kinase (IKK) that catalyzes the phosphorylation of two serines in NF-kB. Once phosphorylated, IkB is degraded and liberates NF-kB to translocate into the nucleus where it binds the promoter regions of many of the same inflammatory genes which lead to its activation. It also upregulates IkB, thus leading to its eventual down regulation [30].
It is well known that one of the main effects of the inflammatory response include cytokines. These are a group of pleiotropic polypeptides (8-26 kDa) that are produced in response to appropriate antigens and regulated the innate and adaptive immune systems. Cytokines are quickly and extensively upregulated in the brain in a variety of disease states [17, 24]. In the brain, cytokines cannot unequivocally be divided into pro- or anti-inflammatory cytokines and are expressed not only in the cells of the immune system, but are also produced by resident brain cells, including neurons and glia [31]. Tumor necrosis factor-α (TNF-α), the interleukins (IL) especially IL-1, IL-4, IL-6, IL-10, and IL-18 and transforming growth factor (TGF)-β are most studied cytokines related to inflammation after acute brain injury. While pro-inflammatory cytokines such as IL-1β and TNF-α, appear to exacerbate brain injury, TGF-β, IL-4, and IL-10 inhibit the expression of pro-inflammatory cytokines and contribute trophic or anti-inflammatory properties [32, 33]. In addition to cytokines, other immune molecules are activated, including adhesion molecules present on the neutrophils and the endothelial cell surface. Adhesion molecules comprise three groups of molecules in various brain injuries: first selectins (P-, E-, and L-isoforms), integrins (LFA-1 and Mac-1) bind to leukocytes, intercellular adhesion molecule-1 and vascular adhesion molecule-1, all of which contribute to inflammatory response by adhesion of leukocytes to activated endothelium, allowing their entry into the CNS [28].
Matrix metalloproteinases (MMPs) represent a family of at least 28 zinc-dependent endopeptidases that break down the extracellular matrix, and can lead to disruption of the blood brain barrier (BBB) leading to further infiltration of circulating immune cells, serum proteins and hemorrhage [34]. After brain injury, they play a central role of immune proteins involved in the inflammatory response. MMPs are proteases which Inactivated MMPs are normally found in the cytosol, but in pathologic states, they can transported extracellularly where they are cleaved to an active form and degrade substrates of the extracellular matrix [17]. MMP-2, -3, and -9 have been described in cerebral ischemia. MMP-9 is first expressed in traditional immune cells after brain injury. Indeed, neutrophils contain and release of MMP-9 [35] and studies of bone marrow chimeras indicates that MMP-9 derived from circulating leukocytes contributes significantly to stroke pathology [36].
Oxidative and nitrosative stresses are an important underlying factor in inflammation response. Reactive oxygen species (ROS) are produced after injured brain cells by various pathway during the reperfusion phase, presumably because their mitochondria are no longer able to neutralize these reactive species [37]. ROS are also generated by inflammatory cells, presumably as a means of host defense against invading pathogens. The increase in ROS can then trigger more immune responses by activating inflammatory transcription factors. Activation of endogenous immune molecules can damage adjacent viable tissue that surround the area of injury [38]. Thus, ROS can participate in a rather vicious cycle of immune response activation and direct cytotoxicity. Similarly, nitrosative stresses include the increase in nitric oxide (NO) via 3 types of nitric oxide synthase (NOS) isoforms: neuronal, endothelial, and inducible (nNOS, eNOS, and iNOS), particularly the iNOS that is expressed after exposure of cells to cytokines and lipopolysaccharide (LPS) [39]. Further, the ROS superoxide and NO can combine to form peroxynitrite, which is a particularly reactive species that is genotoxic as well.
In addition to their better known chaperone properties and anti-apoptotic mechanisms, Hsps are also known to have significant modulating roles in both acting as pro-inflammatory cytokines and mediating regulatory immune responses [40]. Extracellular Hsps are also capable of immunomodulatory functions that trigger immunological responses [41]. While their intracellular chaperone activities appear to prevent apoptosis and stabilize cytoskeletal structures, extracellular Hsps appear to have intercellular signaling functions [42]. Hsp70, perhaps the most studied of the Hsps with respect to its role in inflammation, appears to play dual roles depending on the nature of the stimulus and the ensuing immune response.
In the extracellular environment, Hsps have been well studied in terms of their role in both innate and adaptive immunity where they appear to assist in and potentiate innate and adaptive immune responses. In innate immune responses, Hsp70 can interact with macrophages, microglia, and dendritic cells through TLRs and lead to the activation of the NF-kB transcription factor, which induces pro-inflammatory cytokines production (IL-1β, IL-6, IL-12, and TNF-α) and iNOS [40, 41]. Hsp60, Hsp70, and Hsp90 are all thought to interact with TLR 2 and TLR4 [43, 44]; however, some of this work has been questioned, because some preparations of recombinant Hsps may contain low levels of endotoxin, which is the classic ligand for TLR4 [45]. Extracellular Hsp70 complexed with peptides elicit CD8+ or CD4+ T-cell responses after exogenous administration. Immunization of mice with these same complexes can elicit CD4 responses, indicating that Hsps can act as adjuvant. These Hsp70-peptide complexes can also interact with the macrophage/dendritic cell CD 40, CD91, or LOX-1 receptor and aid in antigen presentation. Extracellular Hsps are also known to participate in the adaptive immune responses (Fig. 1).
As an anti-inflammatory molecule, Hsp70 decreases the release of pro-inflammatory factors such as NF-kB, MMPs, and ROS. Intracellular overexpression of Hsp70 or its intracellular induction by heat stress has been shown to reduce inflammatory cell production of NO and iNOS expression while decreasing NF-kB activation in astrocytes [46]. Heat shock has also been correlated to decreased secretion of TNF-α and reduced generation of ROS. Hsp70 can also prevent responses to inflammatory cytokines such as TNF-α and IL-1 [47], while overexpression of Hsp70 in macrophages blocked LPS-induced increases in TNF, IL-1, IL-10, and IL-12 [48]. Overexpression of Hsp70 was associated with down-regulation of expression of several representative NF-kB dependent pro-inflammatory genes (TNF-α and IL-1β) in experimental stroke [30]. In a model of intracerebral hemorrhage, upregulation of Hsp70 decreased TNF-α expression and attenuated BBB disruption, edema formation, and neurological dysfunction [13].
Heat shock induction of Hsp70 reduces NADPH oxidase activity in neutrophils and increases superoxide dismutase, which scavenges superoxide, in phagocytes [49, 50]. Hsp70 may also affect other proteins and genes known to be involved in inflammatory responses. Others have shown that prior thermal stress leads to inhibition of the inflammatory response, and this inhibition was associated with increased levels of Hsp70 induction and decreased nuclear NF-kB translocation [51, 52]. It has been speculated that Hsp70 could interact with NF-kB's inhibitor protein, IkB, and pre vent IkB phosphorylation and NF-kB dissociation [46]. A few studies have shown that Hsp70 binds to and inhibits NF-kB and/or its regulatory proteins [30, 53], although how it does this may depend on the nature of the stimulus. In a model of TNF-α induced apoptosis, Hsp70 directly inhibited IKK activity, whereas in a model of stroke, Hsp70 appeared to associate with NF-kB and IkB, thus preventing IkB phosphorylation by IKK. The inhibition of NF-kB led to decreased transcription of several immune genes and neuroprotection.
Our labs showed that MMP-9, one of several genes regulated by NF-kB, was reduced in cultured Hsp70-overexpressing astrocytes exposed to ischemia-like insults. Consistent with the notion that Hsp70 may regulate inflammatory protein expression at the transcriptional level, MMP-9 mRNA was also lower in Hsp70-transfected cells. However, Hsp70 expressed in astrocytes seems to not only decrease expression of MMP-9 at both the transcriptional and translational level, it also decreased MMP-2 [54]. Interestingly, MMP-9 expression is regulated by NF-kB, whereas MMP-2 is not, suggesting that Hsp70 may interfere with transcriptional responses in systems other than NF-kB. In fact, studies in alveolar macrophages suggest that heat stress-induced Hsp70 can inhibit STAT1 [55], and STAT1 has been linked to MMP-2 expression [56]. Hsp70 also appears to prevent MMP processing from its pro or inactive form to its cleaved or active form. Thus, it is clear that Hsps have a myriad of roles, some of which modulate immune responses, both adaptive and innate, toward both pro- and anti-inflammatory phenotypes.
In this paper, we have briefly focused on some of the current areas of research on anti-inflammatory role of Hsp70 in brain injury. The immune response pathways arising after acute neurological insults can exacerbate the pathogenic processes of brain, and suppressing inflammation can reduce cell death and improve recovery. Overexpression of Hsp70 in such circumstances appears to be largely anti-inflammatory, where intracellular mechanisms of Hsp70 appear to inhibit innate immune responses. Several studies have demonstrated that overexpression of Hsp70 may have a neuroprotective role in several models of neurodegenerative diseases [16, 41, 57]. Its beneficial effects could be due both to its chaperone role and its ability to protect against various kinds of potentially toxic factors. However, extracellular properties of Hsps appear to act as ligands or co-factors for several pro-inflammatory pathways, both innate and adaptive. Intracellular Hsp70 induction may be a viable approach since there are now several Hsp70 inducing drugs that act by increasing expression at the transcriptional level, and suggests the translatability of such an approach.
Figures and Tables
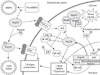 | Fig. 1The mechanism of heat shock protein (Hsp70) modulation adaptive and innate immune signaling pathways following brain injury. There are multiple sites where Hsp70 has been shown to play roles in modulating the inflammatory response. Many extracellular functions appear to potentiate immune responses, whereas intracellular mechanisms appear to be anti-inflammatory. Extracellular Hsp70 complexed with peptides interacts with the CD91 receptor to enhance antigen presentation, while peptidefree Hsp70 acts has been shown to be a ligand for Toll-like receptors (TLR) 2 and 4 to increase immune responses. Interestingly, Hsp70 can also interrupt pro-matrix metalloproteinases (MMPs) cleavage to an active form and lead to reduction in extracellular matrix disruption, which in turn may reduce immune signaling. In a typical immune cell, cytosolic Hsp70 tends to inhibit immune responses. It is tethered in the cytosol by heat shock factor (HSF), but stress and inflammatory signals lead to promote dissociation of Hsp90 from the HSF and phosphorylation (pHSF) allowing pHSF to enter the nucleus, resulting in increased production of Hsp70. Cytosolic Hsp70, presumably through its chaperone activities, has been shown to interact with and inhibit various components of the pro-inflammatory transcription factor nuclear factor kappa B (NF-kB). IKK, IkB, and NF-kB subunits have all been documented to interact with Hsp70. iNOS, inducible nitric oxide synthase. |
Acknowledgements
This work was supported by grants from the National Institutes of Health (NS40516) and the Veteran's Merit Award to MY and a postdoctoral fellowship grant from the American Heart Association to JYK (13POST14810019). The NIH grant to MY and the AHA grant to JYK were administered by the Northern California Institute for Research and Education, and supported by resources of the Veterans Affairs Medical Center, San Francisco, California.
References
1. Georgopoulos C, Welch WJ. Role of the major heat shock proteins as molecular chaperones. Annu Rev Cell Biol. 1993. 9:601–634.
2. Craig EA, Gambill BD, Nelson RJ. Heat shock proteins: molecular chaperones of protein biogenesis. Microbiol Rev. 1993. 57:402–414.
3. Kelly S, Yenari MA. Neuroprotection: heat shock proteins. Curr Med Res Opin. 2002. 18:Suppl 2. s55–s60.
4. Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1988. 22:631–677.
5. Hartl FU, Hayer-Hartl M. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science. 2002. 295:1852–1858.
6. Bukau B, Weissman J, Horwich A. Molecular chaperones and protein quality control. Cell. 2006. 125:443–451.
7. Muchowski PJ, Wacker JL. Modulation of neurodegeneration by molecular chaperones. Nat Rev Neurosci. 2005. 6:11–22.
8. Turturici G, Sconzo G, Geraci F. Hsp70 and its molecular role in nervous system diseases. Biochem Res Int. 2011. 2011:618127.
9. Kim N, Kim JY, Yenari MA. Anti-inflammatory properties and pharmacological induction of Hsp70 after brain injury. Inflammopharmacology. 2012. 20:177–185.
10. Voellmy R. On mechanisms that control heat shock transcription factor activity in metazoan cells. Cell Stress Chaperones. 2004. 9:122–133.
11. Giffard RG, Yenari MA. Many mechanisms for hsp70 protection from cerebral ischemia. J Neurosurg Anesthesiol. 2004. 16:53–61.
12. Adachi H, Katsuno M, Waza M, Minamiyama M, Tanaka F, Sobue G. Heat shock proteins in neurodegenerative diseases: pathogenic roles and therapeutic implications. Int J Hyperthermia. 2009. 25:647–654.
13. Manaenko A, Fathali N, Chen H, Suzuki H, Williams S, Zhang JH, Tang J. Heat shock protein 70 upregulation by geldanamycin reduces brain injury in a mouse model of intracerebral hemorrhage. Neurochem Int. 2010. 57:844–850.
14. Jones Q, Voegeli TS, Li G, Chen Y, Currie RW. Heat shock proteins protect against ischemia and inflammation through multiple mechanisms. Inflamm Allergy Drug Targets. 2011. 10:247–259.
15. Hoshino T, Murao N, Namba T, Takehara M, Adachi H, Katsuno M, Sobue G, Matsushima T, Suzuki T, Mizushima T. Suppression of Alzheimer's disease-related phenotypes by expression of heat shock protein 70 in mice. J Neurosci. 2011. 31:5225–5234.
16. Yenari MA, Liu J, Zheng Z, Vexler ZS, Lee JE, Giffard RG. Antiapoptotic and anti-inflammatory mechanisms of heat-shock protein protection. Ann N Y Acad Sci. 2005. 1053:74–83.
17. Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. J Neuroimmunol. 2007. 184:53–68.
18. Mehta SL, Manhas N, Raghubir R. Molecular targets in cerebral ischemia for developing novel therapeutics. Brain Res Rev. 2007. 54:34–66.
19. Gupta S, Gupta YK, Sharma SS. Protective effect of pifithrin-alpha on brain ischemic reperfusion injury induced by bilateral common carotid arteries occlusion in gerbils. Indian J Physiol Pharmacol. 2007. 51:62–68.
20. Tang XN, Cairns B, Kim JY, Yenari MA. NADPH oxidase in stroke and cerebrovascular disease. Neurol Res. 2012. 34:338–345.
21. Rivest S. Regulation of innate immune responses in the brain. Nat Rev Immunol. 2009. 9:429–439.
22. Ransohoff RM. Immunology: barrier to electrical storms. Nature. 2009. 457:155–156.
23. Zheng Z, Yenari MA. Post-ischemic inflammation: molecular mechanisms and therapeutic implications. Neurol Res. 2004. 26:884–892.
24. Trendelenburg G. Acute neurodegeneration and the inflammasome: central processor for danger signals and the inflammatory response? J Cereb Blood Flow Metab. 2008. 28:867–881.
25. Kim JB, Choi JS, Yu YM, Nam K, Piao CS, Kim SW, Lee MH, Han PL, Park JS, Lee JK. HMGB1, a novel cytokine-like mediator linking acute neuronal death and delayed neuroinflammation in the postischemic brain. J Neurosci. 2006. 26:6413–6421.
26. Qiu J, Nishimura M, Wang Y, Sims JR, Qiu S, Savitz SI, Salomone S, Moskowitz MA. Early release of HMGB-1 from neurons after the onset of brain ischemia. J Cereb Blood Flow Metab. 2008. 28:927–938.
27. Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998. 16:225–260.
28. Yilmaz G, Granger DN. Cell adhesion molecules and ischemic stroke. Neurol Res. 2008. 30:783–793.
29. Barnes PJ, Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997. 336:1066–1071.
30. Zheng Z, Kim JY, Ma H, Lee JE, Yenari MA. Anti-inflammatory effects of the 70 kDa heat shock protein in experimental stroke. J Cereb Blood Flow Metab. 2008. 28:53–63.
31. Lakhan SE, Kirchgessner A, Hofer M. Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J Transl Med. 2009. 7:97.
32. Spera PA, Ellison JA, Feuerstein GZ, Barone FC. IL-10 reduces rat brain injury following focal stroke. Neurosci Lett. 1998. 251:189–192.
33. Zhu Y, Yang GY, Ahlemeyer B, Pang L, Che XM, Culmsee C, Klumpp S, Krieglstein J. Transforming growth factor-beta 1 increases bad phosphorylation and protects neurons against damage. J Neurosci. 2002. 22:3898–3909.
34. Candelario-Jalil E, Yang Y, Rosenberg GA. Diverse roles of matrix metalloproteinases and tissue inhibitors of metal loproteinases in neuroinflammation and cerebral ischemia. Neuroscience. 2009. 158:983–994.
35. Montaner J, Alvarez-Sabín J, Molina C, Anglés A, Abilleira S, Arenillas J, González MA, Monasterio J. Matrix metallo proteinase expression after human cardioembolic stroke: temporal profile and relation to neurological impairment. Stroke. 2001. 32:1759–1766.
36. Gidday JM, Gasche YG, Copin JC, Shah AR, Perez RS, Shapiro SD, Chan PH, Park TS. Leukocyte-derived matrix metalloproteinase-9 mediates blood-brain barrier breakdown and is proinflammatory after transient focal cerebral ischemia. Am J Physiol Heart Circ Physiol. 2005. 289:H558–H568.
37. Sugawara T, Chan PH. Reactive oxygen radicals and pathogenesis of neuronal death after cerebral ischemia. Antioxid Redox Signal. 2003. 5:597–607.
38. Wong CH, Crack PJ. Modulation of neuro-inflammation and vascular response by oxidative stress following cerebral ischemia-reperfusion injury. Curr Med Chem. 2008. 15:1–14.
39. Moncada S. The 1991 Ulf von Euler Lecture. The L-arginine: nitric oxide pathway. Acta Physiol Scand. 1992. 145:201–227.
40. Srivastava P. Roles of heat-shock proteins in innate and adaptive immunity. Nat Rev Immunol. 2002. 2:185–194.
41. Giffard RG, Han RQ, Emery JF, Duan M, Pittet JF. Regulation of apoptotic and inflammatory cell signaling in cerebral ischemia: the complex roles of heat shock protein 70. Anesthesiology. 2008. 109:339–348.
42. Henderson B. Integrating the cell stress response: a new view of molecular chaperones as immunological and physiological homeostatic regulators. Cell Biochem Funct. 2010. 28:1–14.
43. Asea A, Rehli M, Kabingu E, Boch JA, Bare O, Auron PE, Stevenson MA, Calderwood SK. Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem. 2002. 277:15028–15034.
44. Asea A. Heat shock proteins and toll-like receptors. Handb Exp Pharmacol. 2008. (183):111–127.
45. Gaston JS. Heat shock proteins and innate immunity. Clin Exp Immunol. 2002. 127:1–3.
46. Feinstein DL, Galea E, Aquino DA, Li GC, Xu H, Reis DJ. Heat shock protein 70 suppresses astroglial-inducible nitric-oxide synthase expression by decreasing NFkappaB activation. J Biol Chem. 1996. 271:17724–17732.
47. Van Molle W, Wielockx B, Mahieu T, Takada M, Taniguchi T, Sekikawa K, Libert C. HSP70 protects against TNF-induced lethal inflammatory shock. Immunity. 2002. 16:685–695.
48. Ding XZ, Fernandez-Prada CM, Bhattacharjee AK, Hoover DL. Over-expression of hsp-70 inhibits bacterial lipopolysaccharide-induced production of cytokines in human monocyte-derived macrophages. Cytokine. 2001. 16:210–219.
49. Maridonneau-Parini I, Clerc J, Polla BS. Heat shock inhibits NADPH oxidase in human neutrophils. Biochem Biophys Res Commun. 1988. 154:179–186.
50. Polla BS, Stubbe H, Kantengwa S, Maridonneau-Parini I, Jacquier-Sarlin MR. Differential induction of stress proteins and functional effects of heat shock in human phagocytes. Inflammation. 1995. 19:363–378.
51. Guzhova IV, Darieva ZA, Melo AR, Margulis BA. Major stress protein Hsp70 interacts with NF-kB regulatory complex in human T-lymphoma cells. Cell Stress Chaperones. 1997. 2:132–139.
52. Heneka MT, Sharp A, Klockgether T, Gavrilyuk V, Feinstein DL. The heat shock response inhibits NF-kappaB activation, nitric oxide synthase type 2 expression, and macrophage/microglial activation in brain. J Cereb Blood Flow Metab. 2000. 20:800–811.
53. Ran R, Lu A, Zhang L, Tang Y, Zhu H, Xu H, Feng Y, Han C, Zhou G, Rigby AC, Sharp FR. Hsp70 promotes TNF-mediated apoptosis by binding IKK gamma and impairing NF-kappa B survival signaling. Genes Dev. 2004. 18:1466–1481.
54. Lee JE, Kim YJ, Kim JY, Lee WT, Yenari MA, Giffard RG. The 70 kDa heat shock protein suppresses matrix metalloproteinases in astrocytes. Neuroreport. 2004. 15:499–502.
55. Howard M, Roux J, Lee H, Miyazawa B, Lee JW, Gartland B, Howard AJ, Matthay MA, Carles M, Pittet JF. Activation of the stress protein response inhibits the STAT1 signalling pathway and iNOS function in alveolar macrophages: role of Hsp90 and Hsp70. Thorax. 2010. 65:346–353.
56. Johnston JB, Jiang Y, van Marle G, Mayne MB, Ni W, Holden J, McArthur JC, Power C. Lentivirus infection in the brain induces matrix metalloproteinase expression: role of envelope diversity. J Virol. 2000. 74:7211–7220.
57. Zheng Z, Yenari MA. The application of HSP70 as a target for gene therapy. Front Biosci. 2006. 11:699–707.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download