Abstract
To investigate why the development of a completely circular striated sphincter is so rare, we examined histological sections of 11 female and 11 male mid-term human fetuses. In male fetuses, the striated muscle initially extended in the frontal, rather than in the horizontal plane. However, a knee-like portion was absent in the female fetal urethra because, on the inferior side of the vaginal end, a wide groove for the future vestibule opened inferiorly. Accordingly, it was difficult for the developing striated muscle to surround the groove, even though there was not a great difference in width or thickness between the female vestibule and the male urethra. The development of a completely circular striated sphincter seems to be impossible in females because of interruption of the frontal plane by the groove-like vestibule. However, we cannot rule out the possibility that before descent of the vagina, the urethral striated muscle extends posteriorly.
In elderly women, the urethral striated muscle sphincter or rhabdosphincter (RS) does not show a completely circular arrangement, but is located on the pubic and/or lateral side of the urethra [1, 2]. However, Perucchini et al. [3] have quantitatively demonstrated that a thin striated muscle layer remains along the vaginal side of the urethra, especially in young women. A recent detailed study using human female fetuses [4] has shown there is no striated muscle, but a connective tissue "raphe" along the vaginal side of the urethra. This embryology is absolutely consistent with Strasser et al. [1], but seems to rule out the possibility of a vaginal-sided striated muscle sphincter. However, Masumoto et al. [5] have reported that the urethral striated muscle is likely to extend to the vaginal side of the urethra when the vagina merges with the urethra at the higher position: at this stage, the vagina is in the process of descent to the future vestibulum. Thus, the embryological development of the striated sphincter in females is still unclear.
In addition, with a few exceptions, the fetal development of the urethrovaginal sphincter (which is a well developed striated muscle in children and adults [2, 6, 7]) has not been well described [8], and there remains a further possibility that the urethrovaginal sphincter extends posteriorly to surround the thin fetal vagina. In both the fetal urethral and urethrovaginal striated sphincters, we have considered that the topographical anatomy during vaginal descent makes it difficult to understand this aspect of the developmental process. Does the descending vagina disturb the development of a striated sphincter, thus preventing a completely circular arrangement, as stated by Sebe et al. [4]? To address this issue, we examined the fetal topographical anatomy of the urethral RS with the aim of reappraising the existing concept of the difference in its development between the sexes.
The present study was performed in accordance with the provisions of the Declaration of Helsinki 1995 (as revised in Edinburgh 2000). We examined the paraffin-embedded histology of 22 mid-term fetuses (11 males and 11 females). The specimens varied in size and stage: the crown-rump lengths (CRL) of the male specimens were 27, 29, 29, 30, 39, 46, 48, 52, 84, 95, and 103 mm, while those of female specimens were 36, 48, 48, 62, 76, 78, 84, 94, 92, 100, and 103 mm. These sizes corresponded to ovulational ages of approximately 8-13 weeks [9].
All the specimens were part of the large collection kept at the Embryology Institute of the Universidad Complutense, Madrid, and were the products of miscarriages and ectopic pregnancies managed at the Department of Obstetrics of the University. Approval for the study was granted by the university ethics committee (approval number, B-08/374). Because of the nature of the specimens, we were unable to rule out the possibility that they contained pathology. However, no pathology was found in the developing umbilical vessels, liver, intestine, adrenal, and kidney in the specimens examined. Identification of gender was based on observations of the urogenital fold, including the primitive uterus.
After routine procedures for paraffin-embedded histology, most of the specimens were cut almost horizontally (anterior side, tilted inferiorly) at a thickness of 5 µm and at intervals of 50 (100) µm. Sagittal sections were limited to those from male and female fetuses with a CRL of 103 mm, and were cut at a thickness of more than 10 µm because of their large size. Depending on the size of the specimen, 30-200 sections were needed for complete observation, including the entire urethra. Most sections were stained with hematoxylin and eosin, but some were stained with azan (red and blue) or azocarmine (orange).
In all specimens, the vagina had not yet completely descended to its final position in the future vestibulum. In the largest specimen examined (CRL, 103 mm) (Fig. 1A), the inferior end of the vagina reached a point immediately inferior to the pubic symphysis. In the sagittal section, the urethral striated muscle was identified along the pubic side of the urethra, but muscle fibers were unclear because of the direction of muscle fibers. The urethra formed a slight curve at the inferior end of the vagina. In the horizontal section (Fig. 2), the inferior end of the vagina was deeply embedded in the posterior wall of the urethra, and markedly interrupted the urethral wall. The supero-inferior level at which the vagina merged with the urethra appeared to vary between specimens, being evident at a high level on the symphysis pubis (figure not shown), at the mid-height level (Fig. 2C, D) or a low level in the pubic bony arch (Fig. 2A, B). However, these topographical relationships were unrelated to the size of the specimens: e.g., that in Fig. 2A, B (CRL, 48 mm) appears lower than that in Fig. 2C, D (CRL, 100 mm) because, in horizontal section, the pubic bony arch was more widely open in the former than in the latter.
Inferior to the site of vaginal merging with the urethra, the future vaginal vestibule extended along an oblique plane near the anteroposterior axis (Figs. 1A, 2), and accordingly the developing vestibule opened inferiorly. The female vestibular groove was 1.5-fold the thickness of the male urethra at the same age. In horizontal sections, the urethral striated muscle was identifiable even in small specimens, and was attached to the lateral aspect of the vestibule (Fig. 2). The striated muscle was also attached to the anterosuperior aspect of a curved junction between the urethra and the vestibule. In larger specimens (Fig. 2C, D), the inferomedial edges of the levator ani slings were located close to the urethral striated muscle. The vestibular bulb separated the striated muscle from the bulbospongiosus muscle (Fig. 2C, D).
Consequently, the circular striated sphincter was not seen in the female specimens examined. Because of the inferior opening of the developing vestibule, there was no tissue in which the striated muscle was able to extend inferiorly to provide a completely circular arrangement. We did not identify the urethrovaginal sphincter in any of the specimens examined.
Although the present specimens varied in CRL, the urethra consistently showed a change in its direction from a supero-inferior to a postero-anterior course (i.e., a horizontal shift ) at the future prostatic colliculus, i.e., the opening of the Wolffian and Müllerian ducts into the urogenital sinus (Figs. 1B, 3). In addition, on the inferior side of the pubic symphysis, the horizontal urethra again changed direction to the upward course of the penile urethra (Fig. 1B). Throughout the stages examined, the former or proximal knee-like portion was located behind the pubic bony arch. This proximal flexion corresponded to the female curved urethra at the point of merging with the descending vagina.
The future prostatic colliculus and the genital tract were embedded in the posterior wall of the urethra and prominently interrupted the architecture of the urethral wall. This morphology was consistent with the inferior end of the vagina. The urethral striated muscle was identifiable even in small specimens, and was attached to the horizontal urethra on the anterior or distal side of the future prostatic colliculus (Figs. 1B, 3). The flexion or anterior side of the proximal knee-like portion (i.e., contralateral side of the colliculus) was also filled with striated muscle (Fig. 3A, C). However, the striated muscle did not reach the inferior aspect of the urethra, but surrounded the superior 3/4 of the urethra, or almost its entire horizontal course. Notably, the striated muscle did not extend superiorly or proximally beyond the level of the colliculus even at the early stage when the prostate had not yet developed. The inferomedial edges of the levator ani slings did not attach to the striated sphincter, and instead rather loose mesenchymal tissue occupied the space between them (Fig. 3). The bulbospongiosus muscle was located adjacent to the striated sphincter (Fig. 3D). In the 4 large fetuses (CRL, 48, 52, 84, and 95 mm), a membranous structure was evident between the crus penis and the urethral striated muscle.
Consequently, a circular striated sphincter was seen in the male specimens examined (9-13 weeks). There was loose tissue in which the striated muscle was able to extend inferiorly along the horizontal urethra to provide a completely circular arrangement in the later stage. It should be pointed out that the topographical anatomy around the urethra remained constant during the stages examined.
The present study demonstrated that, in both genders, the urethral striated sphincter developed on the inferior or distal side of the site where the genital tracts merged with the urethra. The male fetal urethra changed its direction immediately inferior to the future prostatic colliculus. Thus, the usual model in which the striated muscle surrounds the urethra in the horizontal plane (Fig. 4A) does not correspond to the fetal morphology earlier than 13 weeks, or a CRL of 95 mm. Therefore, at these stages, the future membranous urethra seems to run horizontally along the antero-posterior axis, and the early male striated sphincter extends in the frontal plane (Fig. 4B). In females, by contrast, there was no distal urethra, but the future vestibule opened inferiorly on the inferior side of the vaginal inferior end. Therefore, it appeared impossible for the female urethral striated muscle to "surround" this fissure-like structure corresponding to the future vestibule. Likewise, the urethrovaginal sphincter did not appear to reach the posterior midline area because of the inferiorly opened fetal vestibule. In fact, in adults, the vagina is tightly attached to the posterior wall of the urethra [7, 10, 11]. However, we are unable to rule out the possibility that, before descent of the vagina, the urethral striated muscle extends posteriorly into the space between the vagina and the urethra.
Experiments using an avian chimera system have shown that the perineal muscles originate from myotomal cells in common with the lower extremities [12]. The urethral striated muscle likely originates from a precursor shared with the perineal muscles. In fact, the fetal urethral striated sphincter was located adjacent to the bulbospongiosus muscle, whereas in females, the vestibular bulb later separated the two. Sasaki et al. [13] and Yamaguchi et al. [14], studying mouse embryos, demonstrated an intimate topographical relationship between the bulbospongiosus and the external anal sphincter. The myogenic cells require an attachment or insertion. Mesenchymal tissues around the urethra inferior or distal to the vaginal end, as well as around the anorectum, seem to be favorable for myogenic cell migration and attachment. Conversely, the striated muscle does not provide a specific interfacial tissue for insertion to the vagina, but simply attaches to the latter [15].
In large specimens of both genders, a membranous structure was seen connecting the crus penis or clitoris to the striated sphincter. A similar membranous structure was described by Kato et al. [8] in fetuses at a much later stage than the present specimens. This might represent the earliest form of the urogenital diaphragm or the perineal membrane. Notably, the membranous structure separated the male striated sphincter from the bulbospongiosus, whereas the vestibular bulb played the same role in females. This topographical anatomy is maintained in adults in the urogenital diaphragm [16] and the perineal membrane [8].
A change in topographical anatomy is necessary for transition from the fetal morphology shown in Fig. 4B, C to the adult form: the horizontal male urethra needs to change into the membranous urethra along the supero-inferior axis, while the urethrovaginal sphincter does so along the anteroposterior axis. The developing prostate is likely to contribute significantly to this change in males. In fact, in a CRL 94-mm male fetus with a definite prostate, Oelrich [17] observed an adult-like course of the urethra as well as the striated muscle extending superiorly along the prostate. Thus, the change may occur at a stage slightly later than the present specimens. In contrast, a well-known diagram of the female perineum by Oelrich [6] was based on a 27-year-old woman, and not on observations of fetuses. However, Kato et al. [8] described the urethrovaginal sphincter at 20 weeks (CRL, >180 mm). During the hypothetical topographical change at the late stage, mechanical stress is likely to be responsible for differentiation of the membranous structure between the crus and the striated sphincter (see above) into the male urogenital diaphragm or the female perineal membrane. Subsequently, the female urethral striated muscle seems to extend posterolaterally along the superficial surface of the developed perineal membrane.
Figures and Tables
Fig. 1
Sagittal sections of the fetal urethra and its associated striated muscle (crown-rump lengths, 103 mm). Both (A, female) and (B, male) include a sheet of striated muscle (arrows) restricted to the pubic side of the urethra. The female striated muscle is located at levels superior to the inferior end of the vagina (star in A), while the male muscle attaches to the horizontal urethra proximal to the penile urethra. The female urethra forms a slight curve at the point of merging with the vagina (star in A), while the male urethra clearly changes its direction at the future colliculus as well as on the inferior side of the pubis. A midline fold in the vaginal vestibule (asterisk in A) is the future anterior vaginal column. Hematoxylin and eosin staining. Scale bars=1 mm (A), 5 mm (B).
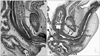
Fig. 2
Almost horizontal sections of the female urethral striated sphincter. (A) and (B; at the same magnification, scale bar in panel A) display a crown-rump lengths (CRL) 48-mm specimen, while (C) and (D; at the same magnification, scale bar in panel C) show a CRL 100-mm specimen. Panel B (D) is located 0.3 (1.2) mm inferior to panel A (C). Thus, a size difference in the superoinferior axis is evident between these two specimens. The vagina (VAG) merges with the urethra (UR) in (A) and (C). In (B) and (D), the future vaginal vestibule is divided into two lumina by a midline fold, i.e., the anterior vaginal column (star). The urethral striated muscle (rhabdosphincter [RS]) attaches to the lateral aspect of the UR and vestibule. The bulbospongiosus muscle (BS) and the RS are separated by the vestibular bulb (VB in panels B and D). Arrow in (C) or (D) indicates a membranous structure connecting the crus of the clitoris (CR) to the RS. This horizontal plane is slightly tilted (see the double-headed arrow in Fig. 3C). C, clitoris; IC, ischiocavernosus muscle; LA, levator ani muscle; OI, obturator internus muscle; P, pubic bone arch; R, rectum; PN, pelvic nerve plexus. Hematoxylin and eosin staining. Scale bars=1 mm (A, C).
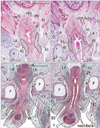
Fig. 3
Almost horizontal sections of the male urethral striated sphincter. Azocarmine (A), azan (B) or hematoxylin and eosin staining (C, D). (A) and (B; at the same magnification, scale bar in panel A) display a crown-rump lengths (CRL) 27-mm specimen, while (C) and (D; at the same magnification, scale bar in panel C) show a CRL 52-mm specimen. Note the distinct difference in size (or scale bar) of these two specimens. Panel B (D), or the inferior aspect of the horizontal urethra, is located 0.1 (0.5) mm inferior to panel A (C), or the superior aspect. The urethral striated muscle (rhabdosphincter [RS]) covers almost all of the horizontal part of the urethra (UR) on the distal side of the colliculus. Both the bulbospongiosus muscle (BS) and the RS are located together along the lateral side of the UR in (D). Arrowheads in all 4 panels indicate loose mesenchymal tissue between the levator ani (LA) and the urethra. Arrows in (C) and (D) indicate a membranous structure connecting between the crus penis (CR) and the RS. This horizontal plane is slightly tilted (see the double-headed arrow in Fig. 3B). ED, ejaculation duct; IC, ischiocavernosus muscle; OI, obturator internus muscle; P, pubic bone arch; PC, peritoneal cavity; PN, pelvic nerve plexus; R, rectum. Scale bars=1 mm (A, C).
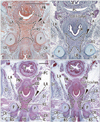
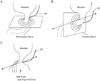
Fig. 4
Schematic representations of the fetal urethral striated muscle. (A) The usual concept of the sphincter extending in a horizontal plane. (B) An actual image of the male sphincter extending in the frontal plane but not reaching the inferior aspect of the urethra. This penile urehra directs inferior in contrast to the fetal morphology. (C) The female sphincter overriding the vaginal vestibule. Double-headed arrows in panels (B) and (C) indicate the tilted horizontal planes shown in Figs. 2 and 3. The vestibule is a narrow and deep groove, but here it is drawn concave to emphasize that it opens inferiorly. In all three forms, the urethral striated muscle is located on the inferior or distal side of the point of merging of the genital tract (GT).
References
1. Strasser H, Ninkovic M, Hess M, Bartsch G, Stenzl A. Anatomic and functional studies of the male and female urethral sphincter. World J Urol. 2000. 18:324–329.
2. Kurihara M, Murakami G, Kajiwara M, Taguchi K, Tsukamoto T, Usui T. Lack of the complete circular rhabdosphincter and a distinct circular smooth muscle layer around the proximal urethra in elderly Japanese women: an anatomical study. Int Urogynecol J Pelvic Floor Dysfunct. 2004. 15:85–94.
3. Perucchini D, DeLancey JO, Ashton-Miller JA, Galecki A, Schaer GN. Age effects on urethral striated muscle. II. Anatomic location of muscle loss. Am J Obstet Gynecol. 2002. 186:356–360.
4. Sebe P, Fritsch H, Oswald J, Schwentner C, Lunacek A, Bartsch G, Radmayr C. Fetal development of the female external urinary sphincter complex: an anatomical and histological study. J Urol. 2005. 173:1738–1742.
5. Masumoto H, Rodríguez-Vázquez JF, Verdugo-López S, Murakami G, Matsubara A. Fetal topographical anatomy of the female urethra and descending vagina: a histological study of the early human fetal urethra. Ann Anat. 2011. 193:500–508.
6. Oelrich TM. The striated urogenital sphincter muscle in the female. Anat Rec. 1983. 205:223–232.
7. Kato M, Matsubara A, Murakami G, Abe S, Ide Y, Sato I, Usui T. Female perineal membrane: a study using pelvic floor semiserial sections from elderly nulliparous and multiparous women. Int Urogynecol J Pelvic Floor Dysfunct. 2008. 19:1663–1670.
8. Kato M, Niikura H, Yaegashi N, Murakami G, Tatsumi H, Matsubara A. Histotopography of the female cavernous nerve: a study using donated fetuses and adult cadavers. Int Urogynecol J Pelvic Floor Dysfunct. 2008. 19:1687–1695.
9. O'Rahilly R, Müller F. Human embryology and teratology. 1996. 2nd ed. New York: Wiley-Liss.
10. Hirata E, Fujiwara H, Hayashi S, Ohtsuka A, Abe S, Murakami G, Kudo Y. Intergender differences in histological architecture of the fascia pelvis parietalis: a cadaveric study. Clin Anat. 2011. 24:469–477.
11. Hirata E, Koyama M, Murakami G, Ohtsuka A, Abe S, Ide Y, Fujiwara H, Kudo Y. Comparative histological study of levels 1-3 supportive tissues using pelvic floor semiserial sections from elderly nulliparous and multiparous women. J Obstet Gynaecol Res. 2011. 37:13–23.
12. Valasek P, Evans DJ, Maina F, Grim M, Patel K. A dual fate of the hindlimb muscle mass: cloacal/perineal musculature develops from leg muscle cells. Development. 2005. 132:447–458.
13. Sasaki C, Yamaguchi K, Akita K. Spatiotemporal distribution of apoptosis during normal cloacal development in mice. Anat Rec A Discov Mol Cell Evol Biol. 2004. 279:761–767.
14. Yamaguchi K, Kiyokawa J, Akita K. Developmental processes and ectodermal contribution to the anal canal in mice. Ann Anat. 2008. 190:119–128.
15. Arakawa T, Murakami G, Nakajima F, Matsubara A, Ohtsuka A, Goto T, Teramoto T. Morphologies of the interfaces between the levator ani muscle and pelvic viscera, with special reference to muscle insertion into the anorectum in elderly Japanese. Anat Sci Int. 2004. 79:72–81.
16. Nakajima F, Takenaka A, Uchiyama E, Hata F, Suzuki D, Murakami G. Macroscopic and histotopographic study of the deep transverse perineal muscle (musculus transversus perinei profundus) in elderly Japanese. Ann Anat. 2007. 189:65–74.
17. Oelrich TM. The urethral sphincter muscle in the male. Am J Anat. 1980. 158:229–246.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download